
Researchers from Kumamoto University studied the cellular aging process in naked mole-rats (NMRs), discovering an NMR-specific “natural senolytic” mechanism that removes senescent cells, potentially contributing to their resistance to aging. The research, utilizing both in vitro and in vivo methods, identified the role of serotonin metabolism and the enzyme MAO in this process, offering insights into anti-aging strategies and treatments against age-related diseases.
Researchers from Japan have discovered a unique species-specific mechanism used by naked mole rats to resist age-related deterioration and diseases.
The naked mole-rat (NMR), or Heterocephalus glaber, is a rodent native to East Africa that boasts a remarkable lifespan of over 37 years. This makes them the longest-lived rodents. Their remarkable resistance to aging and diseases like cancer has piqued the interest of the scientific community, leading researchers hoping to unravel the mechanisms contributing to their longevity.
Understanding Aging in NMRs
Previous studies have explored the role of DNA repair mechanisms, protein stability, and translation accuracy (precise conversion of RNA to proteins) in this regard, but the molecular mechanisms/factors behind their aging resistance remain largely unclear. Moreover, the contribution of cellular senescence to their aging resistance is poorly understood.
Cellular senescence (cellular aging) is characterized by the irreversible arrest of cell division, which progresses with age. Senescent cells are less prone to cell death and accumulate in the tissues as they age, promoting chronic inflammation and compromising the function of these tissues. While cellular senescence plays an important role in aging, little is known about its function in NMRs.

A naked mole rat being bred at Miura Laboratory, Kumamoto University. Credit: Yoshimi Kawamura and Kyoko Miura from Kumamoto University, Japan
Research into Cellular Senescence in NMRs
To this end, a team of researchers from Japan led by Professor Kyoko Miura from the Department of Aging and Longevity Research, Kumamoto University, conducted a series of experiments in vitro and in vivo to understand how cellular senescence occurs in NMRs and if there are any species-specific mechanisms that contribute to suppressing accumulation of senescent cells and their delayed aging. The Department of Aging and Longevity Research, Kumamoto University is the only center in Japan that breeds NMRs and conducts research on their resistance to aging and cancer.
Explaining the rationale behind their study recently published in The EMBO Journal, Professor Miura, states, “Senolysis or the targeted removal of senescent cells has been shown to inhibit aging-related decline in mice. However, whether the findings in mice are generalizable, remains an open question. In this study, we discovered an NMR-specific “natural senolytic” mechanism that may provide an evolutionary rationale for removing senescent cells as a therapeutic strategy to prevent aging.”
Findings and Methodology
The research team used low concentrations of doxorubicin (DXR)—a DNA-damaging agent— to induce cellular senescence in NMR- and mouse-derived skin fibroblasts in vitro. They observed that induction of cellular senescence led to cessation of cell proliferation due to the arrest of the cell cycle with the activation of INK4a and RB (important factors for induction of cellular senescence), in both NMR- and mouse-fibroblasts. However, only NMR cells gradually and significantly activated cell death, suggesting that senescent cell accumulation in NMRs may be suppressed through their removal.
Through further experiments, the researchers observed that there was an accumulation of serotonin (a neurotransmitter that sends signals between nerve cells) in the non-senescent NMR fibroblasts, but not in the mouse fibroblasts. Upon senescence induction, in NMR cells, serotonin was metabolized by monoamine oxidase (MAO; an enzyme highly activated in senescent NMR fibroblasts after induction of cellular senescence) and converted to 5-hydroxyindole acetic acid (5-HIAA; a metabolite), releasing large amounts of hydrogen peroxide (H2O2).
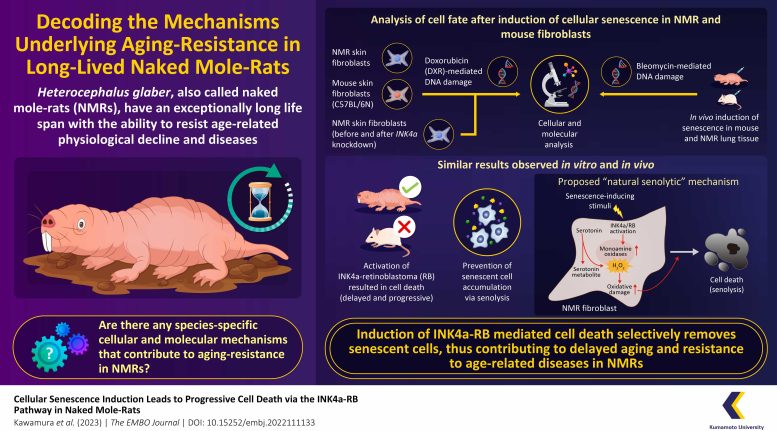
An infographic depicting a unique “natural senolytic” or natural senescent cell removal mechanism identified by Japanese researchers, in naked mole-rats, the longest-lived rodent species. They proposed a species-specific mechanism involving activation of INK4a-RB-signaling and serotonin metabolism, which increases intracellular oxidative damage and causes subsequent death of senescent NMR cells. Credit: Yoshimi Kawamura and Kyoko Miura from Kumamoto University, Japan
The team proposed that oxidative stress due to the intracellular production of H2O2 predisposed the senescent NMR fibroblasts to the cell death pathway, thus leading to senolysis (selective removal of senescent cells). This was confirmed by the observation that the addition of MAO inhibitors and antioxidants inhibited cell death in NMR fibroblasts.
To confirm if a similar mechanism was also prevalent in vivo, the team induced cellular senescence in the lungs of mice and NMRs using bleomycin (a DNA-damaging agent). They observed that cell death, likely due to an acute response to DNA damage, initially increased on day 2 in both mouse and NMR lung cells. However, after an initial rise on day 2, a fall in cell death was observed and by day 21, cell death had increased again, only in NMR lung cells.
Future Directions and Conclusion
Furthermore, treatment with the MAO inhibitor significantly suppressed cell death but increased the number of senescent cells only in the NMR lung on day 21. This suggests that MAO plays a role in inducing cell death and reducing the number of senescent cells following the induction of cellular senescence in NMR lung cells. These results are consistent with the in vitro findings and suggest that MAO contributes to suppressing the accumulation of senescent cells in NMR tissues.
“Further studies focusing on the senescent cell removal mechanism in NMR tissues are needed to understand which kind of senescent cells should be removed, when, and how. Such studies may aid the development of safer and targeted senolytic drugs,” says Prof. Miura while discussing future steps.
Overall, these findings suggest that INK4a-RB-mediated cell death may facilitate the removal of senescent cells in NMRs, helping them resist aging-related degeneration. We are confident that by highlighting a natural senolytic mechanism in this long-lived species, this study would contribute to the development of anti-aging strategies and targeted therapies against age-related diseases such as cancer.
Reference: “Cellular senescence induction leads to progressive cell death via the INK4a-RB pathway in naked mole-rats” by Yoshimi Kawamura, Kaori Oka, Takashi Semba, Mayuko Takamori, Yuki Sugiura, Riyo Yamasaki, Yusuke Suzuki, Takeshi Chujo, Mari Nagase, Yuki Oiwa, Shusuke Fujioka, Sayuri Homma, Yuki Yamamura, Shingo Miyawaki, Minoru Narita, Takaichi Fukuda, Yusuke Sakai, Takatsugu Ishimoto, Kazuhito Tomizawa, Makoto Suematsu, Takuya Yamamoto, Hidemasa Bono, Hideyuki Okano and Kyoko Miura, 11 July 2023, The EMBO Journal.
DOI: 10.15252/embj.2022111133
The study was funded by the Japan Science and Technology Agency, the Japan Agency for Medical Research and Development, the Japan Society for the Promotion of Science, the Takeda Science Foundation, the Mitsubishi Foundation, the Kanzawa Medical Research Foundation, the Japan Foundation for Aging and Health, the KOSE Cosmetology Research Foundation, the Princess Takamatsu Cancer Research Fund, the Nakatomi Foundation, the Naito Foundation, the Foundation for Promotion of Cancer Research, the Kato Memorial Bioscience Foundation, the MSD Life Science Foundation, the Inamori Foundation, the SGH Foundation, the Terumo Foundation for Life Sciences and Arts, the Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care, and the Frontier Salon Foundation.








Be the first to comment on "Nature’s Anti-Aging Blueprint: How Naked Mole-Rats Are Redefining What We Know About Aging"