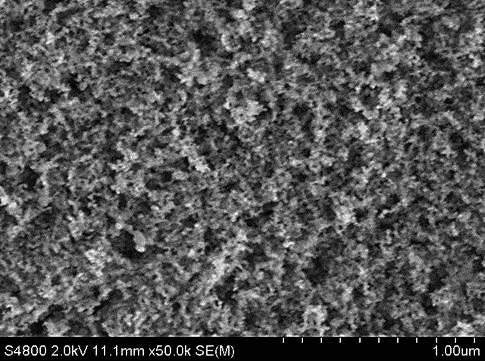
A scanning electron microscope image of a new superhydrophobic material shows the rough surface of functionalized alumina nanoparticles. Scientists at Rice University and the University of Swansea led the creation of the environmentally friendly material. Credit: University of Swansea
Scientists at Rice University have developed a new class of superhydrophobic nanomaterials that might simplify the process of protecting surfaces from water.
A material made by scientists at Rice University, the University of Swansea, the University of Bristol, and the University of Nice Sophia Antipolis is inexpensive, nontoxic, and can be applied to a variety of surfaces via spray- or spin-coating.
The researchers led by Rice chemist Andrew Barron reported their findings in the American Chemical Society journal ACS Applied Materials and Interfaces.
The hydrocarbon-based material may be a “green” replacement for costly, hazardous fluorocarbons commonly used for superhydrophobic applications, Barron said.
“Nature knows how to make these materials and stay environmentally friendly,” Barron said. “Our job has been to figure out how and why, and to emulate that.”
The lotus leaf was very much on their minds as the researchers tried to mimic one of the most hydrophobic — water-repelling — surfaces on the planet. Barron said the leaf’s abilities spring from its hierarchy of microscopic and nanoscale double structures.
“In the lotus leaf, these are due to papillae within the epidermis and epicuticular waxes on top,” he said. “In our material, there is a microstructure created by the agglomeration of alumina nanoparticles mimicking the papillae and the hyperbranched organic moieties simulating the effect of the epicuticular waxes.”
Fabrication and testing of what the researchers call a branched hydrocarbon low-surface energy material (LSEM) were carried out by lead author Shirin Alexander, a research officer at the Energy Safety Research Institute at the Swansea University Bay Campus.
There, Alexander coated easily synthesized aluminum oxide nanoparticles with modified carboxylic acids that feature highly branched hydrocarbon chains. These spiky chains are the first line of defense against water, making the surface rough. This roughness, a characteristic of hydrophobic materials, traps a layer of air and minimizes contact between the surface and water droplets, which allows them to slide off.
To be superhydrophobic, a material has to have a water contact angle larger than 150 degrees. The contact angle is the angle at which the surface of the water meets the surface of the material. The greater the beading, the higher the angle. An angle of 0 degrees is basically a puddle, while a maximum angle of 180 degrees defines a sphere just touching the surface.
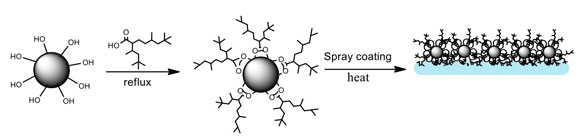
An environmentally friendly superhydrophobic coating repels water as effectively as commercial coatings that employ hazardous materials, according to scientists at Rice University and the University of Swansea. Credit: Shirin Alexander/University of Swansea
The Barron team’s LSEM, with an observed angle of about 155 degrees, is essentially equivalent to the best fluorocarbon-based superhydrophobic coatings, Barron said. Even with varied coating techniques and curing temperatures, the material retained its qualities, the researchers reported.
Potential applications include friction-reducing coatings for marine applications where there is an international agreement in trying to keep water safe from such potentially dangerous additives as fluorocarbons, Barron said. “The textured surfaces of other superhydrophobic coatings are often damaged and thus reduce the hydrophobic nature,” he said. “Our material has a more random hierarchical structure that can sustain damage and maintain its effects.”
He said the team is working to improve the material’s adhesion to various substrates, as well as looking at large-scale application to surfaces.
Co-authors of the paper are Julian Eastoe, a professor of chemistry at the University of Bristol; Alex Lord, a researcher at the Swansea University Center for Nanohealth; and Frédéric Guittard, a professor at the University of Nice Sophia Antipolis. Barron is the Charles W. Duncan Jr.–Welch Professor of Chemistry a professor of materials science and nanoengineering at Rice and the Sêr Cymru Chair of Low Carbon Energy and Environment at Swansea.
The Robert A. Welch Foundation and the Welsh Government Sêr Cymru Program supported the research.
Reference: “Branched Hydrocarbon Low Surface Energy Materials (LSEMs) for Superhydrophobic Nanoparticle Derived Surfaces” by Shirin Alexander, Julian Eastoe, Alex M. Lord, Frédéric Guittard and Andrew R. Barron, 7 December 2015, ACS Applied Materials & Interfaces.
DOI: 10.1021/acsami.5b09784






Be the first to comment on "Scientists Develop a New Class of Superhydrophobic Nanomaterials"