
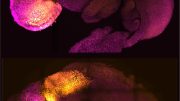
Using a microscopy image and graphic rendering, the artists illustrate a minimal synthetic cell that can sense a directional chemical cue and self-organize in response. Credit: Inoue Lab at Johns Hopkins Medical Institute, created by Shiva Razavi and Turhan Pathan, edited
Scientists have engineered a synthetic cell capable of symmetry breaking, responding to chemical signals similar to immune cells. This advancement at Johns Hopkins could lead to targeted drug delivery systems, utilizing the cell’s ability to move towards and release drugs at specific sites.
Scientists at Johns Hopkins Medicine have developed a minimal synthetic cell capable of following external chemical signals and exhibiting a fundamental biological concept known as “symmetry breaking.” This incredible innovation aims to enhance our understanding of cellular movement and devise new methods for transporting drugs within the body.
The research is detailed today (June 12) in the journal Science Advances.
Understanding Symmetry Breaking
A step that precedes the movement of a cell, symmetry breaking, happens when a cell’s molecules, which are initially arranged symmetrically, reorganize into an asymmetric pattern or shape, usually in response to stimuli. This is similar to how migrating birds break symmetry when they shift into a new formation in response to an environmental compass like sunlight or landmarks. On a microscopic level, immune cells sense chemical signals concentrated at an infection site and break symmetry to traverse a blood vessel wall to reach the infected tissue. As cells break symmetry, they transform into polarized and asymmetric structures that prepare them to move toward their target.
“The notion of symmetry breaking is crucial to life, impacting fields as diverse as biology, physics, and cosmology,” says Shiva Razavi, Ph.D., who led the research as a graduate student at Johns Hopkins and is now a postdoctoral fellow at Massachusetts Institute of Technology. “Understanding how symmetry breaking works is key to unlocking the fundamentals of biology and discovering how to harness this information to devise therapeutics.”
Finding ways to mimic and control symmetry breaking in synthetic cells has long been considered essential for understanding how cells can survey their chemical environment and rearrange their chemical profile and shape in response.
Development of a Synthetic Protocell
For this study scientists created a giant vesicle with a double-layered membrane —a bare-bones, simplified synthetic cell or protocell made of phospholipids, purified proteins, salts and ATP that provides energy. With its spherical shape, the protocell is nicknamed “the bubble.” In their experiments, the scientists successfully engineered the protocell with a chemical-sensing ability that prompts the cell to break symmetry, changing from a nearly perfect sphere to an uneven shape. The system was specifically designed to mimic the first step in an immune response, able to signal for neutrophils to attack germs based on proteins they sense around them, the researchers say.
“Our study demonstrates how a cell-like entity can sense the direction of an external chemical cue, mimicking the conditions you would find in a living organism,” Razavi says. “By building a cell-like structure from scratch, we can better identify and understand the essential components required for a cell to break symmetry in its most simplified form.”
Future Applications in Drug Delivery
One day chemical sensing could be used for targeted drug delivery within the body, the scientists say.
“The idea is that you can package anything you want into these bubbles — protein, RNA, DNA, dyes or small molecules — tell the cell where to go using chemical sensing, and then have the cell burst near its intended target so that a drug can be released,” says senior author Takanari Inoue, Ph.D., professor of cell biology and director of the Center for Cell Dynamics at Johns Hopkins Medicine.
Engineering of Chemical Sensing Mechanisms
To activate the vesicle’s chemical-sensing ability, researchers planted two proteins that act as molecular switches — called FKBP and FRB — within the synthetic cell. The protein FKBP was placed in the center of the cell, while FRB was planted on the membrane. When the scientists introduced a chemical — rapamycin — outside of the bubble cell, FKBP moved to the membrane to bind with FRB, triggering a process called actin polymerization, or a reorganization of the synthetic cell’s skeleton.
Inside the protocell, the chemical reaction resulted in a rod-like structure made up of actin that put pressure on the cell membrane, bending it.
Imaging Techniques and Future Directions
The researchers used a specialized type of rapid 3D imaging called confocal microscopy to record the protocell’s chemical-sensing ability; they had to record images quickly, at a rate of one frame per every 15 to 30 seconds, as the protocells responded quickly to the chemical signal.
Up next, the researchers aim to equip these synthetic cells with the ability to move toward a desired target. Ultimately, researchers hope to engineer synthetic cells that could have significant potential applications in targeted drug delivery, environmental sensing and other areas where precise movement and response to stimuli are crucial.
Reference: “Synthetic control of actin polymerization and symmetry breaking in active protocells” by Shiva Razavi, Felix Wong, Bedri Abubaker-Sharif, Hideaki T. Matsubayashi, Hideki Nakamura, Nhung Thi Hong Nguyen, Douglas N. Robinson, Baoyu Chen, Pablo A. Iglesias and Takanari Inoue, 12 June 2024, Science Advances.
DOI: 10.1126/sciadv.adk9731
Other scientists who contributed to this research include Bedri Abubaker-Sharif, Hideaki T. Matsubayashi, Hideki Nakamura, Nhung Thi Hong Nguyen, Douglas N. Robinson, and Pablo A. Iglesias of Johns Hopkins; Felix Wong from Massachusetts Institute of Technology; and Baoyu Chen of Iowa State University.
Funding for this research was provided by the National Institutes of Health (5R01GM123130, R01GM136858, R35GM149329, R35GM128786, R01GM149073, R01GM66817 and S10OD016374), the Department of Defense Advanced Research Projects Agency (HR0011-16-C- 0139), the National Science Foundation and the PRESTO program of the Japan Science and Technology Agency.








Be the first to comment on "Synthetic Wonders: How Johns Hopkins Is Redefining Cellular Engineering"