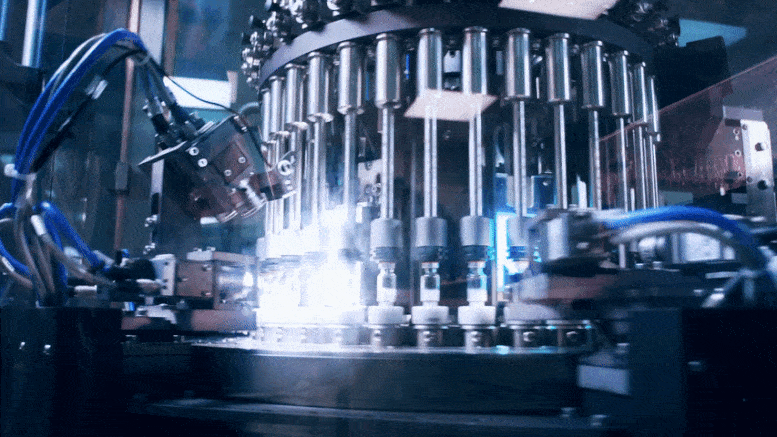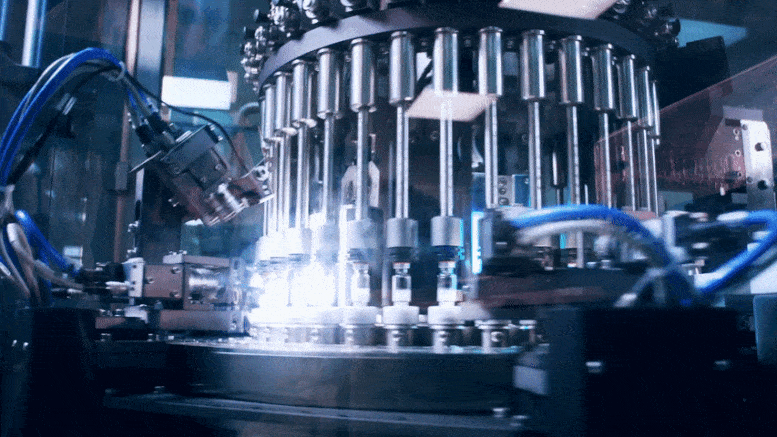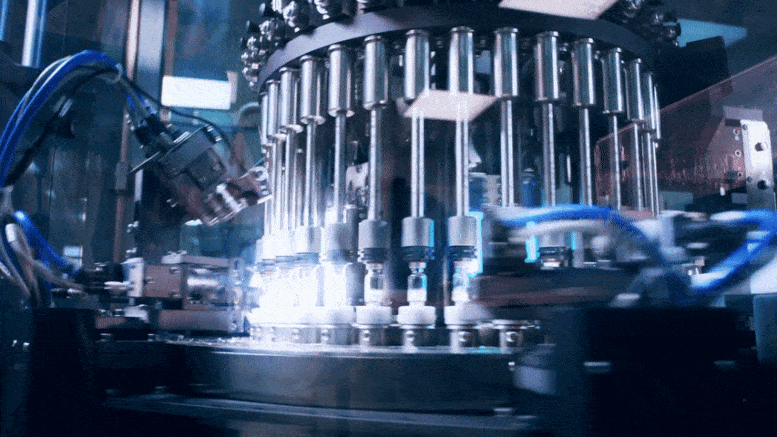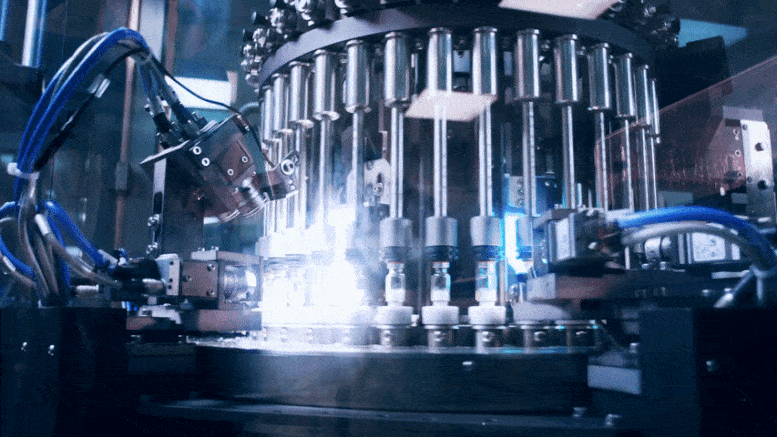
To rapidly produce a large number of COVID-19 vaccines, companies need to share not only information on what to make but also the knowledge of how to make it.
Massive, rapid production of vaccines to fight COVID-19 will require firms to share know-how not just about what to make, but how to make it, write Nicholson Price and colleagues in a new Policy Forum published in the journal Science. They cite the recent approval granted by the U.S. Department of Justice to six pharmaceutical firms to exchange “technical information” on manufacturing processes related to monoclonal antibody (mAb) candidates as an example — one that might pave the way for standardizing manufacturing of biologics going forward.
As the world rushes to identify safe and effective vaccines and therapeutics to counter the COVID-19 epidemic, attention is turning to the next step: manufacturing these products at enormous scale. This might require, in some cases, companies to make products originally developed by other firms, in which case they may need to know that company’s manufacturing methods.
For myriad reasons, however, patents on products like vaccines often fail to disclose necessary manufacturing information. “[M]aintaining pervasive secrecy for manufacturing COVID-19 vaccines during the pandemic could cause dramatic failure,” Price and colleagues argue. They say that relevant information for quick and effective scale-up must be readily available. Especially when the products that will ultimately be made at scale are as-yet unidentified, broader efforts to ensure their eventual scalability should be a focus.
The authors acknowledge that transferring knowledge may not be trivial, including in cases where knowledge has not been codified. However, several entities might facilitate this type of knowledge transfer, they say, including existing international organizations and national governments.
Reference: “Knowledge transfer for large-scale vaccine manufacturing” by W. Nicholson Price II, Arti K. Rai and Timo Minssen, 13 August 2020, Science.
DOI: 10.1126/science.abc9588





Be the first to comment on "What’s Required for Massive, Rapid Production of COVID-19 Vaccines"