A Northwestern University study introduces a cost-effective catalyst made from molybdenum and table sugar that converts CO2 into carbon monoxide, presenting a viable method to transform captured carbon into useful products like fuel precursors.
New catalyst may provide a potential solution for utilizing captured carbon.
A new catalyst made from an inexpensive, abundant metal and common table sugar has the power to destroy carbon dioxide (CO2) gas.
In a new Northwestern University study, the catalyst successfully converted CO2 into carbon monoxide (CO), an important building block to produce a variety of useful chemicals. When the reaction occurs in the presence of hydrogen, for example, CO2 and hydrogen transform into synthesis gas (or syngas), a highly valuable precursor to producing fuels that can potentially replace gasoline.
With recent advances in carbon capture technologies, post-combustion carbon capture is becoming a plausible option to help tackle the global climate change crisis. But how to handle the captured carbon remains an open-ended question. The new catalyst potentially could provide one solution for disposing of the potent greenhouse gas by converting it into a more valuable product.
The study will be published in the May 3 issue of the journal Science.
“Even if we stopped emitting CO2 now, our atmosphere would still have a surplus of CO2 as a result of industrial activities from the past centuries,” said Northwestern’s Milad Khoshooei, who co-led the study. “There is no single solution to this problem. We need to reduce CO2 emissions and find new ways to decrease the CO2 concentration that is already in the atmosphere. We should take advantage of all possible solutions.”
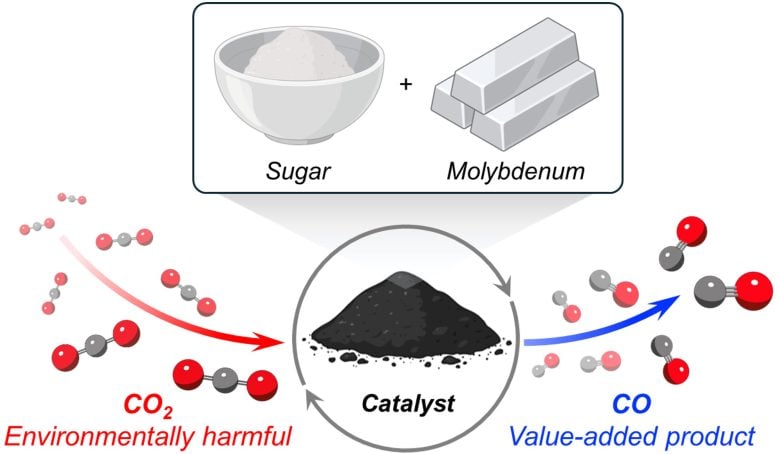
This schematic shows the full process of creating the catalyst and using it to convert carbon dioxide. Credit: Milad Khoshooei
“We’re not the first research group to convert CO2 into another product,” said Northwestern’s Omar K. Farha, the study’s senior author. “However, for the process to be truly practical, it necessitates a catalyst that fulfills several crucial criteria: affordability, stability, ease of production, and scalability. Balancing these four elements is key. Fortunately, our material excels in meeting these requirements.”
An expert in carbon capture technologies, Farha is the Charles E. and Emma H. Morrison Professor of Chemistry at Northwestern’s Weinberg College of Arts and Sciences. After starting this work as a Ph.D. candidate at the University of Calgary in Canada, Khoshooei now is a postdoctoral fellow in Farha’s laboratory.
Solutions from the pantry
The secret behind the new catalyst is molybdenum carbide, an extremely hard ceramic material. Unlike many other catalysts that require expensive metals, such as platinum or palladium, molybdenum is an inexpensive, non-precious, Earth-abundant metal.
To transform molybdenum into molybdenum carbide, the scientists needed a source of carbon. They discovered a cheap option in an unexpected place: the pantry. Surprisingly, sugar — the white, granulated kind found in nearly every household — served as an inexpensive, convenient source of carbon atoms.
“Every day that I tried to synthesize these materials, I would bring sugar to the lab from my home,” Khoshooei said. “When compared to other classes of materials commonly used for catalysts, ours is incredibly inexpensive.”
Successfully selective and stable
When testing the catalyst, Farha, Khoshooei, and their collaborators were impressed by its success. Operating at ambient pressures and high temperatures (300-600 degrees Celsius), the catalyst converted CO2 into CO with 100% selectivity.
High selectivity means that the catalyst acted only on the CO2 without disrupting surrounding materials. In other words, industry could apply the catalyst to large volumes of captured gases and selectively target only the CO2. The catalyst also remained stable over time, meaning that it stayed active and did not degrade.
“In chemistry, it’s not uncommon for a catalyst to lose its selectivity after a few hours,” Farha said. “But, after 500 hours in harsh conditions, its selectivity did not change.”
This is particularly remarkable because CO2 is a stable — and stubborn — molecule.
“Converting CO2 is not easy,” Khoshooei said. “CO2 is a chemically stable molecule, and we had to overcome that stability, which takes a lot of energy.”
Tandem approach to carbon clean-up
Developing materials for carbon capture is a major focus of Farha’s laboratory. His group develops metal-organic frameworks (MOFs), a class of highly porous, nano-sized materials that Farha likens to “sophisticated and programmable bath sponges.” Farha explores MOFs for diverse applications, including pulling CO2 directly from the air.
Now, Farha says MOFs and the new catalyst could work together to play a role in carbon capture and sequestration.
“At some point, we could employ a MOF to capture CO2, followed by a catalyst converting it into something more beneficial,” Farha suggested. “A tandem system utilizing two distinct materials for two sequential steps could be the way forward.”
“This could help us answer the question: ‘What do we do with captured CO2?’” Khoshooei added. “Right now, the plan is to sequester it underground. But underground reservoirs must meet many requirements in order to safely and permanently store CO2. We wanted to design a more universal solution that can be used anywhere while adding economic value.”
Reference: “An active, stable cubic molybdenum carbide catalyst for the high-temperature reverse water-gas shift reaction” by Milad Ahmadi Khoshooei, Xijun Wang, Gerardo Vitale, Filip Formalik, Kent O. Kirlikovali, Randall Q. Snurr, Pedro Pereira-Almao and Omar K. Farha, 2 May 2024, Science.
DOI: 10.1126/science.adl1260
The study was supported by the U.S. Department of Energy, the National Science Foundation and the Natural Sciences and Engineering Research Council of Canada.









Great story about molybdenum carbide suger catalyst for CO2. Keep us posted!
What is the benefit of such a process if it just ends up producing precursors to fuel, particularly fuels that would replace gasoline? Wouldn’t a fuel like that automatically involve combining carbon with oxygen that would produce carbon dioxide, putting the whole thing back at square one from a carbon dioxide in the atmosphere point of view?
I’m at a loss to see the benefit of making fuels out of carbon dioxide pulled from the atmosphere. It seems the whole point of getting carbon dioxide out of the atmosphere is to keep it out of the atmosphere. If instead all we get is a net zero change in carbon dioxide, what’s the point?
Hi Matt . I’m an active environmental campaigner who is extremely serious about trying to stop fossil fuel use. I too am skeptical about Carbon capture technologies, particularly since they are currently,and may well remain,incapable of bringing about reductions in CO2 on the scale at which we need to achieve them. BUT there would ,in fact be a huge difference between using CO2 captured from the atmosphere to produce fuel and using fossil fuels. The issue with fossil fuels is that we are releasing millions of years worth of stored Carbon ( largely in the form of CO2) over a ,geologically speaking , very short time period. That is what is increasing temperatures. If we could just keep cycling our CO2 it wouldn’t be such a problem ( though we should still try to reduce current atmospheric levels ). The issue for me with these technologies is that ( currently at least) they are not applicable at the scale we need them. But that’s no reason not to try…
There is a fundamental, dangerous stupidity here. The image, for example, frames CO2 as environmentally harmful. So while everyone is blabbering about why too much CO2 will kill us all, few people seem to bother trying to understand the other side of that coin and advocate for seeking the proper balance rather than treating carbon dioxide as a poison. I have seen this stupidity in my own country where some years ago an “environmental minister” started talking about how bad it was that carbon poisons our soil, before being pulled to the side for a quick word.
Let’s zoom out a little, drop some of the assumptions, and look at the full role of carbon dioxide in the environment. Too little CO2 in the atmosphere will reduce plant growth worldwide and reduce agricultural yields. It will reduce the greenhouse effect until the planet is too cold.
Everything must exist in balance.
There is a natural carbon cycle on this planet, and you know what falls into it? All life. Your body. You are a carbon-based lifeform. Carbon is not bad. Imbalance is bad. We need to change the discourse so that we do not run off a bloody cliff with this insanity.
Demonize the elemental foundation of life itself at your peril. That said, controlling the element that determines how many plants can grow and therefore controlling all life on this planet sounds nice and convenient to certain agendas, doesn’t it?
Wake up. Ditch the rhetoric. Look at the issue more carefully. Don’t be bullied into turning off your brain and turning into a drooling mess whose most nuanced scientific thought is “carbon baaaad”. Acknowledge its role in all life and stop promoting the narrative that it’s something fundamentally harmful and scary.
Too little CO2 is a problem, but too much CO2 even more so. Not just for the climate, but for the oceans which will become more acidic if CO2 levels rise. So yes, look at the issue more carefully.
I totally agree. Everybody forgot the basics from elementary school. The life on Earth, the densest and most thriving flora and fauna, happened to be during dinosaur era – and CO2 was tenfold today’s.
If the level of CO2 in your blood is too low, you experience blood acidosis and the body enters into a panic attack. Instant remedy is CO2 saturation by re-inhaling the exhaled air.
Scientists, do not rob us of essential life element.
The ambient level of CO2 in the atmosphere is approximately 420 PPM. At 150 PPM of CO2 the rate of photosynthesis would slow down and plants would start to die. At 100 PPM all plant Life would die, which means all people and animals would die of hunger. At 30000 ppm some people will start to have problems breathing, 40000 ppm people would start to die, and 50000 ppm of CO2 all people would die. Why do we need to lower the C02 levels again???
So wait… The idea is to take carbon dioxide (a non-poisonous emission – one you exhale with every breath) and convert it into carbon monoxide (a highly toxic emission – which is what comes out of your vehicle’s exhaust)? I’m failing to see where this makes any damn sense.
It’s almost funnier than that. They correctly point out that carbon monoxide is useful for a lot of chemical applications and even as a heating fuel. The funny part is that pretty much without exception everything we use it for converts it back to carbon dioxide. So, this would be expending energy and resources (a net negative effect on the environment) to create a catalyst (that is less effective than the means we already have to make carbon monoxide as a product), in order to convert carbon dioxide to caron monoxide which is then used in processes that change it back into carbon dioxide. The net effect is whatever waste energy and cost there is in producing the catalyst and process to capture and ship the CO compared to how it’s already sourced. No upside at all and certainly additional negative environmental effect in energy cost alone.
One suspects that agendas are in play here with the whole carbon campaign. But then using the air like an open sewer….
It has been a long tim sinde i did chemistry but here i go:
the reason why we produce CO2 in the first place is because of we need to get energy
and CO2 is the product of burning combustible.
if we need to SPEND energy to convert it “back” from CO2 to CO
where would the energy come from ???
it s a bit like not burning fuel in the first place…
also CO itself is not that much better for us to manage
and as said above if it is then used back as source of energy (combustible)
then the whole cycle is then totally pointless…
“Environmentally harmful CO2” said no experiment or study ever.
So what you’re doing here is adding a ton of energy to a reaction so you can replicate photosynthesis and end up with vegetable oil. Why not just let nature do it for free? We’ve been carbon negative this entire time through the natural process anyways (like it matters).
Of course, 2LoT steps in and you’re going to be burning more fuel for the amount of fuel you produce, creating more CO2 than you convert.
Such a non sense should not be published. Where is the benefit of such research?? Convrting CO2 into Co using of huge amount of energy.
Ok
There are a lot of things wrong with this article. How exactly is a catalyst accomplishing the reduction of CO2 to CO? What is being oxidized? And what else would be there to react with whatever matter that is being oxidized? How would a catalyst overcome the thermodynamics of the reaction that would lead to the formation of CO?
I was a Humanities major, but I “get” this from just High School chemistry. 2(CO2)=>2(CO)+O2. “Ambient pressure” means sea level air pressure. Heat the ambient air gases by running them through small glass pipes under focused sunlight. Nothing but the CO2 gets touched. The carbon monoxide thus produced goes into a refinery, to make other products (fuel). Burning the fuel produces more CO2, which gets recycled over and over again. It’s like growing trees then burning the wood, over and over–a circular system–only the catalyst works faster, and it never wears out.
Wow, denialists a go-go today. Get in the garden if you’re going to conc. solar drive the catalyst unsuitably; decoking it at 750°C is a fine sentiment, but it’s so hot you’ll kind of end up consuming the O2 again and blowing dioxane into the feedstock. Run the cool green chemistry in the synth loop and get ketones or kerosene fuel or polyarene epoxides or biodegradable valorated lignin monomers. If you make more than 80 billion metric tons, consider how carbon-neutral the mideast has become (cough) and slow it down in a few years so the plants have enough CO2.