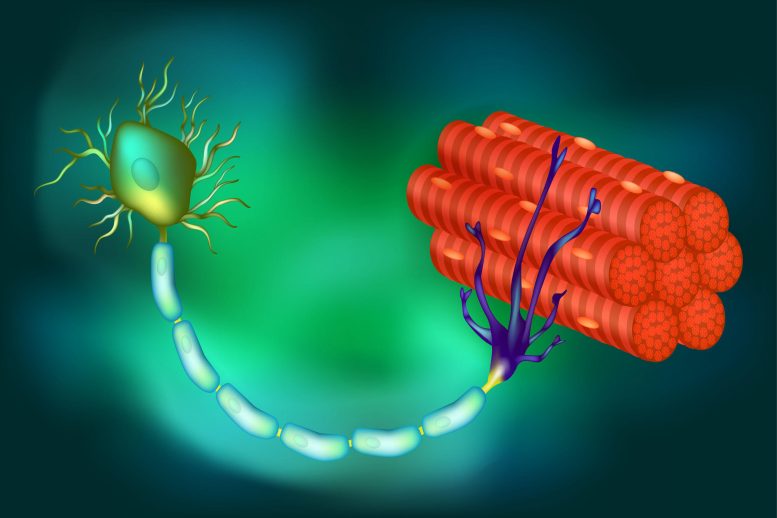
An illustration of the connections between muscle fibers and a motor neuron, the latter of which relies on crucial proteins to create and send signals to the fibers to contract, resulting in movement. In some diseases, such as ALS, those proteins are lost, the neurons die, and paralysis results. Credit: Somatic Movement Center
In research involving both mice and humans, a genetically-engineered medication was found to replenish the levels of a critical protein, thereby preserving the function of motor neurons. This breakthrough, specifically relevant to amyotrophic lateral sclerosis where this function is typically compromised, could potentially pave the way for clinical trials.
In nearly all cases of amyotrophic lateral sclerosis (ALS) and in as many as half of all Alzheimer’s disease (AD) and frontotemporal dementia incidents, a protein known as TDP-43 is misplaced from its usual position in the cell’s nucleus. This displacement results in the loss of stathmin-2, a protein that is vital for the regeneration of neurons and the preservation of their links to muscle fibers, both of which are essential for muscle contraction and movement.
In a study published in the journal Science, a group of researchers, led by Don Cleveland, Ph.D., who is a Distinguished Professor of Medicine, Neurosciences, and Cellular and Molecular Medicine at the University of California San Diego School of Medicine, reveal that the loss of stathmin-2 can be reversed. This was achieved using specially engineered DNA drugs that reinstate the normal processing of RNA that encodes proteins.
“With mouse models, we engineered to misprocess their stathmin-2 encoding RNAs, like in these human diseases, we show that administration of one of these designer DNA drugs into the fluid that surrounds the brain and spinal cord restores normal stathmin-2 levels throughout the nervous system,” Cleveland said.

Don Cleveland, Ph.D., Distinguished Professor of Medicine, Neurosciences, and Cellular and Molecular Medicine at UC San Diego School of Medicine, is among the most highly cited researchers in the world for his work investigating neurodegenerative diseases. Credit: Erik Jepsen, UC San Diego
Cleveland is broadly credited with developing the concept of designer DNA drugs, which act to either turn on or turn off genes associated with many degenerative diseases of the aging human nervous system, including ALS, AD, Huntington’s disease, and cancer.
Several designer DNA drugs are currently in clinical trials for multiple diseases. One such drug has been approved to treat a childhood neurodegenerative disease called spinal muscular atrophy.
The new study builds upon ongoing research by Cleveland and others regarding the role and loss of TDP-43, a protein associated with ALS, AD, and other neurodegenerative disorders. In ALS, TDP-43 loss impacts the motor neurons that innervate and trigger the contraction of skeletal muscles, causing them to degenerate, eventually resulting in paralysis.
“In almost all instances of ALS, there is aggregation of TDP-43, a protein that functions in maturation of the RNA intermediates that encode many proteins. Reduced TDP-43 activity causes misassembly of the RNA-encoding stathmin-2, a protein required for maintenance of the connection of motor neurons to muscle,” said Cleveland.
“Without stathmin-2, motor neurons disconnect from muscle, driving paralysis that is characteristic of ALS. What we have now found is that we can mimic TDP-43 function with a designer DNA drug, thereby restoring correct stathmin-2 RNA and protein level in the mammalian nervous system.”
Specifically, the researchers edited genes in mice to contain human STMN2 gene sequences and then injected antisense oligonucleotides — small bits of DNA or RNA that can bind to specific RNA molecules, blocking their ability to make a protein or changing how their final RNAs are assembled — into cerebral spinal fluid. The injections corrected STMN2 pre-mRNA misprocessing and restored stathmin-2 protein expression fully independent of TDP-43 function.
“Our findings lay the foundation for a clinical trial to delay paralysis in ALS by maintaining stathmin-2 protein levels in patients using our designer DNA drug,” Cleveland said.
Reference: “Mechanism of STMN2 cryptic splice-polyadenylation and its correction for TDP-43 proteinopathies” by Michael W. Baughn, Ze’ev Melamed, Jone López-Erauskin, Melinda S. Beccari, Karen Ling, Aamir Zuberi, Maximilliano Presa, Elena Gonzalo-Gil, Roy Maimon, Sonia Vazquez-Sanchez, Som Chaturvedi, Mariana Bravo-Hernández, Vanessa Taupin, Stephen Moore, Jonathan W. Artates, Eitan Acks, I. Sandra Ndayambaje, Ana R. Agra de Almeida Quadros, Paayman Jafar-nejad, Frank Rigo, C. Frank Bennett, Cathleen Lutz, Clotilde Lagier-Tourenne and Don W. Cleveland, 16 March 2023, Science.
DOI: 10.1126/science.abq5622


Be the first to comment on "ALS Breakthrough: New DNA Treatment Could Delay Paralysis"