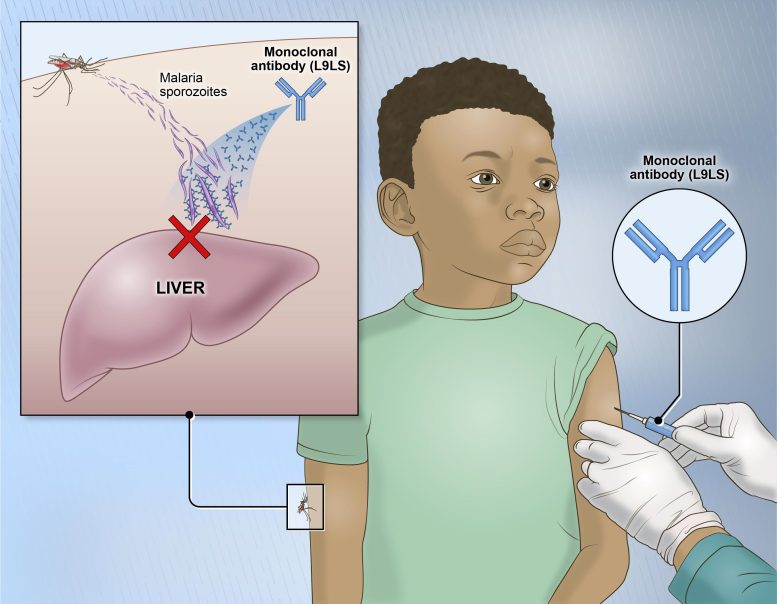
A single injection of an experimental monoclonal antibody called L9LS prevented malaria infection in children in Mali. L9LS binds to and neutralizes “sporozoites,” the form of the malaria parasite transmitted by mosquitoes that invades the liver to initiate infection. Credit: NIH
A new study reports a single dose of a malaria antibody was 77% effective in protecting children in Mali from malaria, marking a significant step in malaria prevention efforts.
One injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children in Mali during the country’s six-month malaria season, according to the results of a mid-stage clinical trial. The trial assessed an investigational monoclonal antibody developed by scientists at the National Institutes of Health (NIH). The results will be published today (April 26) in The New England Journal of Medicine.
“A long-acting monoclonal antibody delivered at a single health care visit that rapidly provides high-level protection against malaria in these vulnerable populations would fulfill an unmet public health need,” said Dr. Jeanne Marrazzo, director of the National Institute of Allergy and Infectious Diseases, part of NIH.
In 2022, the P. falciparum parasite caused a majority of the nearly 250 million estimated cases of malaria globally and most of the more than 600,000 malaria deaths, according to the World Health Organization. Most malaria cases and deaths are among children in Africa. Malaria parasites such as P. falciparum are transmitted to people by mosquito bites.
Clinical Trial Results
The clinical trial assessed two dose levels, with 19% of the 300mg-dose group and 28% of the 150mg-dose group developing symptomatic malaria, providing protective efficacy of 77% and 67% against symptomatic malaria, respectively. Among children who received placebo, 81% became infected with Plasmodium falciparum, and 59% had symptomatic malaria during the six-month study period.
The authors note that the trial demonstrated for the first time that a single dose of a monoclonal antibody given by subcutaneous injection can provide high-level protection against malaria in children in an area of intense malaria transmission.
Development of the Antibody
In 2020, scientists at NIAID’s Vaccine Research Center reported that they had isolated the antibody from a volunteer who had been vaccinated with an experimental malaria vaccine. The antibody was modified with a mutation that prolonged its durability in the bloodstream following administration. In an earlier study, conducted in Mali by the same research group, a previously discovered antibody was highly protective against P. falciparum infection in adults when given intravenously. However, the new antibody was shown to be more potent in animal studies and was manufactured at a higher concentration than CIS43LS, allowing it to be given by subcutaneous injection.
The trial in Mali took place in two parts, first to assess safety in a small number of adults and children, and then in a larger clinical efficacy trial involving 225 children. The efficacy trial took place from July 2022 to January 2023 and included healthy children 6 to 10 years of age, 75 of whom received a 300 mg dose, 75 a 150 mg dose, and 75 of whom received a placebo.
Future Research Directions
The researchers are continuing clinical development of the experimental antibody, focusing on other high-risk populations, such as infants and young children, children hospitalized with severe anemia, and pregnant women. An ongoing clinical trial in Kenya is assessing the efficacy of the antibody in children 5 months to 5 years of age over a 12-month study period, and scientists are also conducting a clinical trial in Mali to assess the antibody in women of childbearing potential to prepare to test the antibody in pregnancy.
Reference: “Subcutaneous Administration of a Monoclonal Antibody to Prevent Malaria” by Kassoum Kayentao, Aissata Ongoiba, Anne C. Preston, Sara A. Healy, Zonghui Hu, Jeff Skinner, Safiatou Doumbo, Jing Wang, Hamidou Cisse, Didier Doumtabe, Abdrahamane Traore, Hamadi Traore, Adama Djiguiba, Shanping Li, Mary E. Peterson, Shinyi Telscher, Azza H. Idris, William C. Adams, Adrian B. McDermott, Sandeep Narpala, Bob C. Lin, Leonid Serebryannyy, Somia P. Hickman, Andrew J. McDougal, Sandra Vazquez, Matthew Reiber, Judy A. Stein, Jason G. Gall, Kevin Carlton, Philipp Schwabl, Siriman Traore, Mamadou Keita, Amatigué Zéguimé, Adama Ouattara, M’Bouye Doucoure, Amagana Dolo, Sean C. Murphy, Daniel E. Neafsey, Silvia Portugal, Abdoulaye Djimdé, Boubacar Traore, Robert A. Seder and Peter D. Crompton, 1 May 2024, New England Journal of Medicine.
DOI: 10.1056/NEJMoa2312775
NIAID led the clinical trial in conjunction with the University of Sciences, Techniques and Technologies of Bamako, Mali, through NIAID’s Division of Intramural Research International Centers of Excellence in Research (ICER) program. For more details about the clinical trial, see ClinicalTrials.gov using identifier NCT05304611.








Be the first to comment on "Experimental NIH Antibody Protects Children From Malaria in Clinical Trial"