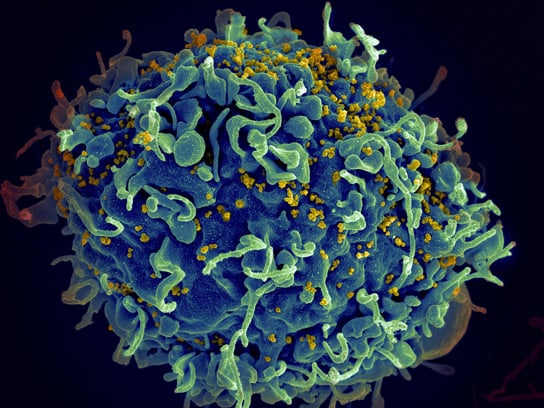
Scientists aim to create an HIV vaccine that induces the production of “broadly neutralizing” antibodies, capable of neutralizing multiple strains of the virus and preventing infection.
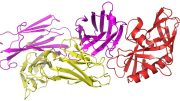
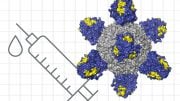
A team of researchers has found HIV-1 broadly neutralizing antibody precursor B cells that are present in most people, and has described the design of an HIV vaccine germline-targeting immunogen capable of binding those B cells.
Some people infected with HIV naturally produce antibodies that effectively neutralize many strains of the rapidly mutating virus, and scientists are working to develop a vaccine capable of inducing such “broadly neutralizing” antibodies that can prevent HIV infection.
An emerging vaccine strategy involves immunizing people with a series of different engineered HIV proteins as immunogens to teach the immune system to produce broadly neutralizing antibodies against HIV. This strategy depends on the ability of the first immunogen to bind and activate special cells, known as broadly neutralizing antibody precursor B cells, which have the potential to develop into broadly neutralizing antibody-producing B cells.
A research team has now found that the right precursor (“germline”) cells for one kind of HIV broadly neutralizing antibody are present in most people, and has described the design of an HIV vaccine germline-targeting immunogen capable of binding those B cells. The findings by scientists from The Scripps Research Institute (TSRI), the International AIDS Vaccine Initiative (IAVI), and the La Jolla Institute for Allergy and Immunology were published in Science.
“We found that almost everybody has these broadly neutralizing antibody precursors, and that a precisely engineered protein can bind to these cells that have the potential to develop into HIV broadly neutralizing antibody-producing cells, even in the presence of competition from other immune cells,” said the study’s lead author, William Schief, TSRI professor and director, Vaccine Design of the IAVI Neutralizing Antibody Center at TSRI, in whose lab the engineered HIV vaccine protein was developed.
The body’s immune system contains a large pool of different precursor B cells so it can respond to a wide variety of pathogens. But that also means that precursor B cells able to recognize a specific feature on a virus surface are exceedingly rare within the total pool of B cells.
“The challenge for vaccine developers is to determine if an immunogen can present a particular viral surface in a way that distinct B cells can be activated, proliferate, and be useful,” said study co-author Shane Crotty, professor at the La Jolla Institute. “Using a new technique, we were able to show—well in advance of clinical trials—that most humans actually have the right B cells that will bind to this vaccine candidate. It is remarkable that protein design can be so specific as to ’find’ one in a million cells, demonstrating the feasibility of this new vaccine strategy.”
The work offers encouraging insights for a planned Phase 1 clinical trial to test a nanoparticle version of the engineered HIV vaccine protein, the “eOD-GT8 60mer.” “The goal of the clinical study will be to test the safety and the ability of this engineered protein to elicit the desired immune response in humans that would look like the start of broadly neutralizing antibody development,” Schief said. “Data from this new study was also important for designing the clinical trial, including the size and the methods of analysis.”
In June, scientists from TSRI, IAVI, and The Rockefeller University reported that the eOD-GT8 60mer produced antibody responses in mice that showed some of the traits necessary to recognize and inhibit HIV. If the eOD-GT8 60mer performs similarly in humans, additional boost immunogens are thought to be needed to ultimately induce broadly neutralizing antibodies that can block HIV.
The new work also provides a method for researchers to assess whether other new vaccine proteins can bind their intended precursor B cells. This method is a valuable tool in the design of more targeted and effective vaccines against AIDS, providing the ability to vet germline-targeting immunogens before testing them in large, time-consuming, and costly clinical trials.
Looking at blood donated by healthy volunteers, the scientists found B cells that were capable of creating “VRC01-class” antibodies that recognized a critical surface patch, or epitope, of HIV. VRC01-class broadly neutralizing antibodies are a group of antibodies isolated from different individuals that appear to have developed in a very similar way, and it has been hypothesized that the starting VRC01-class B cells were very similar in the different people. The eOD-GT8 60mer is designed to engage these precursor B cells to initiate HIV broadly neutralizing antibody development.
Other contributors to the paper, “HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen,” included Joseph Jardine, Daniel Kulp, Colin Havenar-Daughton, Anita Sarkar, Bryan Briney, Devin Sok, Fabian Sesterhenn, June Ereno-Orbea, Oleksandr Kalyuzhniy, Isaiah Deresa, Xiaozhen Hu, Skye Spencer, Meaghan Jones, Erik Georgeson, Jumiko Adachi, Michael Kubitz, Allan decamp, Jean-Philippe Julien, Ian Wilson, and Dennis Burton. This work was supported by the International AIDS Vaccine Initiative Neutralizing Antibody Consortium and Center; the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology and Harvard; the Bayer Science and Education Foundation; the Helen Hay Whitney Foundation; Howard Hughes Medical Institute; Bill & Melinda Gates Foundation; and the National Institute of Allergy and Infectious Diseases (P01 AI094419, Center for HIV/AIDS Vaccine Immunology & Immunogen Discovery (CHAVI-ID) 1UM1AI100663, P01 AI82362 and R01 AI084817.)
Reference: “HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen” by Joseph G. Jardine, Daniel W. Kulp, Colin Havenar-Daughton, Anita Sarkar, Bryan Briney, Devin Sok, Fabian Sesterhenn, June Ereño-Orbea, Oleksandr Kalyuzhniy, Isaiah Deresa, Xiaozhen Hu, Skye Spencer, Meaghan Jones, Erik Georgeson, Yumiko Adachi, Michael Kubitz, Allan C. Decamp, Jean-Philippe Julien, Ian A. Wilson, Dennis R. Burton, Shane Crotty and William R. Schief, 25 March 2016, Science.
DOI: 10.1126/science.aad9195


Be the first to comment on "New Findings Provide a Design for an HIV Vaccine Germline-Targeting Immunogen"