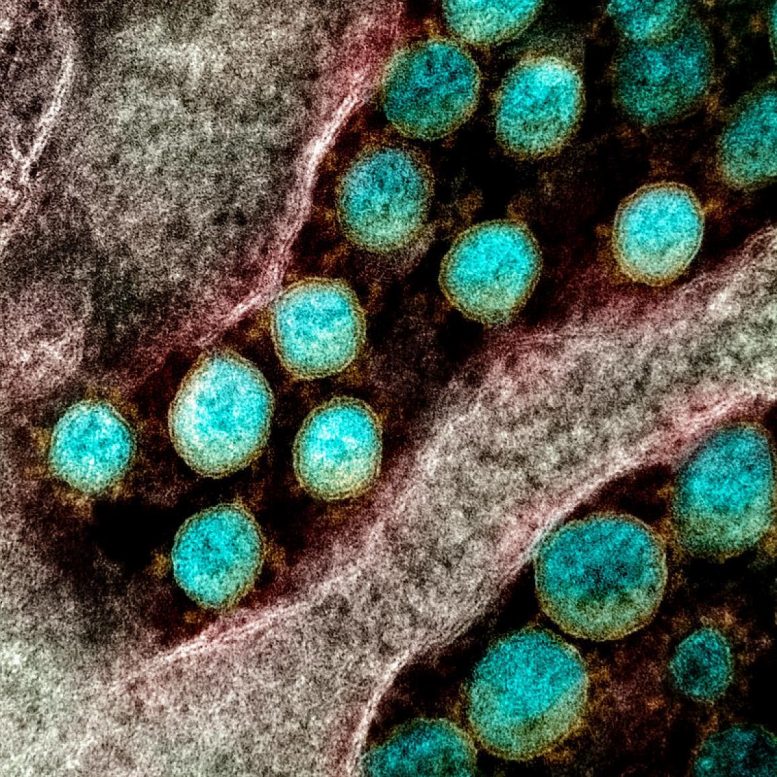
Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
Study enrolling adults with mild to moderate COVID-19 in the United States.
A clinical trial has begun to evaluate whether the malaria drug hydroxychloroquine, given together with the antibiotic azithromycin, can prevent hospitalization and death from coronavirus disease 2019 (COVID-19). The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, is sponsoring the trial, which is being conducted by the NIAID-funded AIDS Clinical Trials Group (ACTG). Teva Pharmaceuticals is donating medications for the study.
The Phase 2b trial will enroll approximately 2,000 adults at participating ACTG sites across the United States. Study participants must have confirmed infection with SARS-CoV-2, the virus that causes COVID-19, and be experiencing fever, cough and/or shortness of breath. The investigators anticipate that many of those enrolled will be 60 years of age or older or have a comorbidity associated with developing serious complications from COVID-19, such as cardiovascular disease or diabetes. Participants will be randomly assigned to receive short-term treatment with either hydroxychloroquine and azithromycin or matching placebos. People living with HIV and pregnant and breastfeeding women also are eligible to participate in the study. The first participant enrolled today in San Diego, California.
“We urgently need a safe and effective treatment for COVID-19. Repurposing existing drugs is an attractive option because these medications have undergone extensive testing, allowing them to move quickly into clinical trials and accelerating their potential approval for COVID-19 treatment,” said NIAID Director Anthony S. Fauci, M.D. “Although there is anecdotal evidence that hydroxychloroquine and azithromycin may benefit people with COVID-19, we need solid data from a large randomized, controlled clinical trial to determine whether this experimental treatment is safe and can improve clinical outcomes.”
As of May 17, the World Health Organization (WHO) has reported 4.53 million cases of and 307,395 deaths from COVID-19 worldwide. In the United States, 1.44 million confirmed COVID-19 cases and 87,315 deaths have been reported as of May 17, according to the Centers for Disease Control and Prevention (CDC).
Currently, there are no specific therapeutics approved by the U.S. Food and Drug Administration to treat people with COVID-19. Hydroxychloroquine is FDA-approved to prevent and treat malaria, as well as to treat the autoimmune diseases rheumatoid arthritis and lupus. Some preliminary reports have suggested that hydroxychloroquine, alone or in combination with the FDA-approved antibiotic azithromycin, may benefit people with COVID-19. Numerous clinical trials are planned or underway, including a recently launched study supported by NIH’s National Heart, Lung and Blood Institute evaluating the safety and effectiveness of hydroxychloroquine for the treatment of adults hospitalized with COVID-19. On March 28, FDA issued an Emergency Use Authorization (EUA) to allow hydroxychloroquine and medical-grade chloroquine to be distributed from the Strategic National Stockpile and prescribed by doctors to hospitalized adolescents and adults with COVID-19, as appropriate, when a clinical trial is not available or feasible.
Participants in the ACTG study, called A5395, will receive oral medications to take at home. Those randomly assigned to the experimental treatment group will take 400 milligrams (mg) of hydroxychloroquine twice on the first day and 200 mg twice daily for an additional six days. They also will take 500 mg of azithromycin on the first day and 250 mg daily for an additional four days. The control group will receive equivalent numbers of placebo pills. Neither the participants nor the study team will know who received experimental treatment or placebo until the end of the trial.
Participants will record their symptoms, adherence to treatment, and major events such as hospitalizations in a diary for 20 days. Study staff will follow up with participants by telephone during this period. When possible, participants will come to the clinical research site for an in-person visit at day 20. Additional follow-ups will be conducted by telephone three and six months after treatment starts.
The main objective of the study is to determine whether hydroxychloroquine and azithromycin can prevent hospitalization and death due to COVID-19. Additionally, investigators will evaluate the safety and tolerability of the experimental treatment for people with SARS-CoV-2 infection. While hydroxychloroquine and azithromycin are both considered safe in most people, they can cause side effects ranging from headache and nausea to, rarely, heart rhythm problems that can be life-threatening. Because of the risk of heart problems when hydroxychloroquine is used alone or combined with azithromycin, FDA cautions that use of hydroxychloroquine for COVID-19 should be limited to clinical trials or for treating certain hospitalized patients under EUA so clinicians can monitor patients for adverse effects.
“This study will provide key data to aid responses to the COVID-19 pandemic,” said ACTG Chair Judith Currier, M.D., of the University of California, Los Angeles. “We are pleased to be able to leverage ACTG’s existing infrastructure for HIV treatment clinical trials to quickly implement this important study.”
The study team is led by Protocol Chair Davey Smith, M.D., of the University of California, San Diego. David Wohl, M.D., of the University of North Carolina at Chapel Hill, and Kara W. Chew, M.D., and Eric S. Daar, M.D., both of the University of California, Los Angeles, serve as protocol vice-chairs. The trial is expected to enroll quickly given the high incidence of COVID-19, and initial results may be available later this year.
For more information about A5395, visit ClinicalTrials.gov and search identifier NCT04358068. Adults interested in participating in the study should email [email protected](link sends e-mail).
NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing, and treating these illnesses.





Why do you continue to try and prove hydroxychloroquine is expedient in the treatment of COVID-19. The side affects of the drug are significant. See https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2020/72885a-eng.php
Worldwide case fatality rate for patients taking HC is 0.5% (N=5335). Compare that to 7.1% case fatality worldwide or 10.6% for areas that don’t allow HC treatment early (like New York). Side effects of the drug that’s been taken by nearly a billion people including pregnant women are rare and don’t compare to the upside.
This!
Glad to see this study happening. Seems 2 months late. Wish it included ZINC now that we have seen the NYU preprint study that shows HZ+AZ+ZINC cutting deaths in half compared with HZ+AZ without ZINC. The NYU study was observational of hospitalized patients. What I would really like to see is a study of HC+AZ+ZINC given very early, at disease onset, to at-risk patients. This is when anti-viral treatments do their best work.
Typo. HZ == HC.
I agree with Tom Maz. Why aren’t they including zinc? From what I understand, the hydroxy “opens the door” to the virus and the zinc kills/inactivates it. That is what Dr. Zelenko used and it worked according to what has been published. Could you pose the question to the people in charge of the study?
Are there any trials of just the hydroxychloroquine. I know that was used in China and it is being used where I live in Costa Rica. Costa Rica, with a population of 5 million. has 830 positive cases, 600 recovered and a death toll of 10. They have been giving hydroxychloroquine to all people testing positive or suspected. Hard to argue with their outcomes
Exactly. It’s not just Costa Rica either. Bahrain, India, Turkey, South Korea, Senegal, China, and many other countries are reporting tremendous success using HC treatment early (not after hospitalization!). In fact, I’m not aware of any country that is using HC early that has an above-average case fatality rate. Countries that are late to the HC bandwagon are also having great success (Italy, Brazil). The outcomes do seem to speak for themselves but the RCT cult would rather let people suffer while they wait for the perfect experiment.
This is a waste that will probably kill more people. Hydroxychlororquine hasn’t worked for any viruses.
MedCram #34 shows the mechanism of using HCQ. It facilitates zinc to enter the cell and attach to the virus to reduce its harm. This trial seems not to
include zinc and seems destined to fail. I believe this is the objective so to not have any alternative than vaccine. Someone plans to make a lot of money on the vaccine.
I agree with the comments that zinc should be included. There are two theories as to why chloroquine analogs work on viruses. One is that they change the pH (acidity) inside the bubbles of cell membrane that transport virus bits. By doing so, the virus’ replication doesn’t work.
The other is that they attract zinc across the cell membranes as an “ionophore”. If the ionophore hypothesis is correct, then for chlorquine analogs to work, you need a ready supply of zinc in the body.
I don’t understand. It’s clearly been shown that it’s the ZINC that’s working as the main anti-viral with the HCQ acting in this very important role as being an Ionophore.
Absolutely. This study will be useless if it is not designed for early onset; and to include ZINC. It is as if Fauci is designing this to fail.
You’re assuming two things, that 1 – that’s the only theoretical effect HCQ has as an antiviral, and 2 – everyone is zinc deficient.
I have been studying the effects of this drug, and although I disagree with the President on most things, I heartily agree with him on this. The drug works best with supplemental zinc and an antibiotic. It allows a crossover of cells, normally with a lipid barrier, to stop the virus replicating using zinc. I have read many positive things. But there is a shortage of HCQ. Lots of others are using it already for as Lupus, etc. It is not an expensive therapy either. However, this study did not name zinc as part of the package. Good luck anyway!
Everyone who commented about Zinc being an important adjunct with HCQ is correct in their Zinc opinion. This study without zinc will bot be a strong positive. The doctors who proposed the study and those who approved it have failed and are wasting our money. This study is a WASTE OF TIME and Money as designed.
Confirmation Bias is conspicuously being applied to sabotage HUMAN trials deliberately engineered to fail by producing misleading results ignoring the synergism requiring Zinc. Political motivation has subverted SCIENCE to design HUMAN trials intended to produce misleading results useful to be an artifice for FRAUD. What would be more helpful and ETHICAL is HUMAN trials that are intelligent and HONEST. Along with Zinc it is possible Selenium and other immune system requirements may be other synergistic factors.