Osaka University researchers identify RSPO3/LGR4 proteins that reduce inflammation, promote neural growth, and improve sensory and motor functions in mice after ischemic stroke, offering a promising avenue for new stroke therapies.
Researchers from Osaka University identify a protein that reduces inflammation, stimulates neural growth, and improves sensory and motor functions following ischemic stroke in mice.
Scientists have discovered that proteins R-spondin 3 (RSPO3) and LGR4 can reduce inflammation and promote neural growth in mice following ischemic stroke. By injecting RSPO3 into the brains of mice 24 and 48 hours after stroke, the researchers observed reduced sensory and motor deficits, decreased pro-inflammatory factors, and increased neurite outgrowth. This study suggests that targeting the RSPO3/LGR4 signaling pathway could offer a promising avenue for developing new therapies for ischemic stroke and improving patient outcomes.
Ischemic stroke, caused by a blockage of blood flow to the brain, is a common cause of death and disability. Treatments are urgently needed to improve patient outcomes, because recovery currently depends largely on the timely injection of a blood clot-dissolving drug. Priorities for therapy include limiting inflammation at the ischemic site and rebuilding neuronal connections damaged by the stroke. However, a molecule that can achieve these therapeutic effects has remained elusive.
In a study to be published today (May 11, 2023) in the journal Stroke, researchers from Osaka University provide new hope for patients. They have identified two proteins, R-spondin 3 (RSPO3) and LGR4, that trigger a cascade of reactions in cells (i.e., a signaling pathway) to reduce inflammation in the ischemic brain. RSPO3 and LGR4 also stimulate the growth of extensions from neurons, a process called neurite outgrowth.
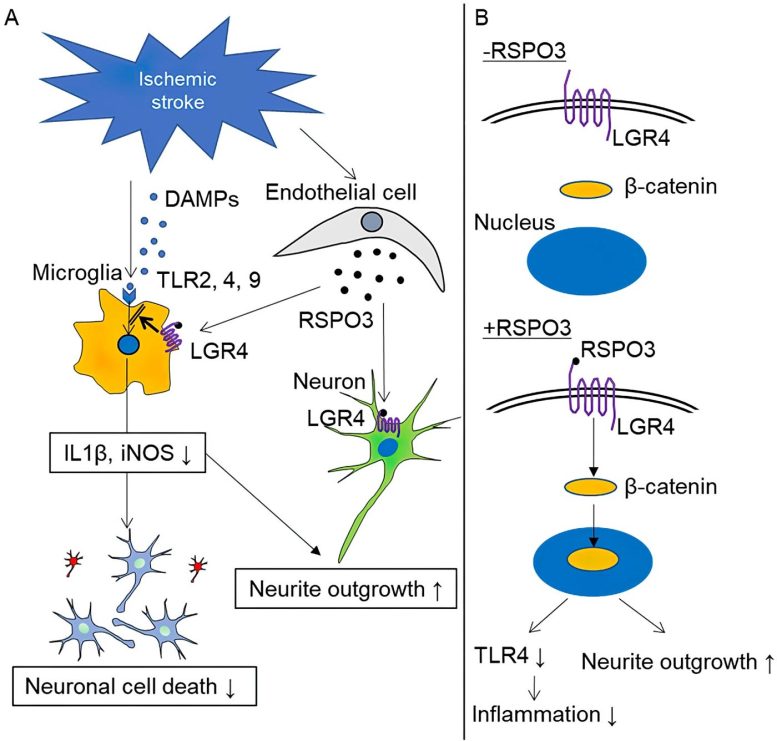
Administered RSPO3 and RSPO3 secreted by endothelial cells promote neurite outgrowth and neuroprotection by suppressing TLR2, TLR4, and TLR9-induced inflammation in microglia and by acting directly on neurons (A). RSPO3 stimulates nuclear translocation of β-catenin, resulting in the downregulation of TLR4 expression and promotion of neurite outgrowth (B). Credit: Stroke in Press
“Previous studies showed that RSPO3 was beneficial in lung injuries caused by inflammation. We also knew that RSPO3 stimulates a signaling pathway, named the ‘canonical Wnt pathway’, that promotes neurite outgrowth,” explains Munehisa Shimamura, lead author of the study. “We wondered whether RSPO3 reduces inflammation and promotes neurite outgrowth after ischemic stroke.”
Previous studies have shown that RSPO3 and LGR4 are present in the same brain structures, and that RSPO3 activates LGR4 to stimulate the canonical Wnt pathway. The team from Osaka University localized RSPO3 in endothelial cells and LGR4 in microglia/macrophage cells and neurons in the ischemic brain.
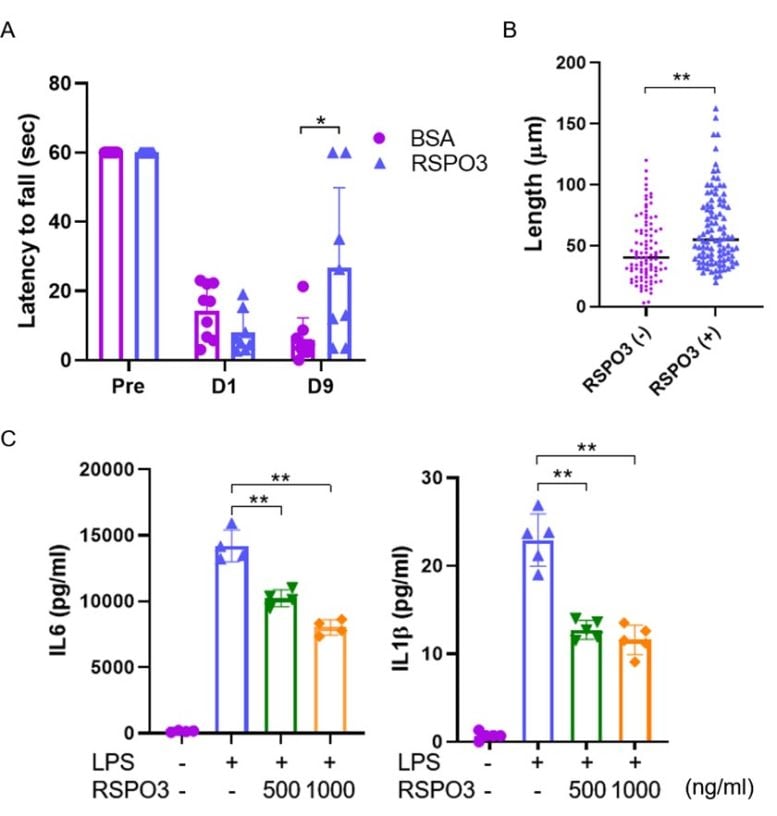
(A) When recombinant RSPO3 was administered intracerebroventricularly one day after the stroke, the neurological deficit was reduced. (B) Neurite outgrowth was promoted in primary neuronal cells cultured in medium containing RSPO3. (C) RSPO3 suppressed the expression of inflammatory cytokines in LPS-stimulated microglia. Credit: Modified from Stroke
“Because of this close localization, RSPO3 could act on LGR4,” explains Hironori Nakagami, a senior author of the study. “To test this hypothesis, we injected RSPO3 into the brains of mice 24 and 48 hours after ischemic stroke.”
Remarkably, nine days after the stroke, mice that were injected with RSPO3 exhibited fewer sensory and motor deficits than mice injected with a control protein. The expression of pro-inflammatory factors was reduced, whereas signs of neurite outgrowth increased. How? The researchers found that RSPO3/LGR4 decreased the expression of TLR4, which is one of proteins essential for inducing inflammation.
These findings are particularly exciting because RPSO3 was given to mice one day after the stroke, suggesting a potential benefit to treatments in later stages of stroke. Thus, targeting RSPO3/LGR4 signaling is a promising lead for developing new therapies for ischemic stroke and improving patient outcomes.
Reference: “R-spondin 3/LGR4 axis is a novel inflammatory and neurite outgrowth signaling system in the ischemic brain in mice” by Munehisa Shimamura, Hiroki Hayashi, Nan Ju, Shota Yoshida, Satoshi Baba, Tsutomu Sasaki, Hideki Mochizuki, Ryuichi Morishita and Hironori Nakagami, 11 May 2023, Stroke.
DOI: 10.1161/STROKEAHA.122.041970


Be the first to comment on "Novel Proteins Offer Hope for Improved Stroke Recovery"