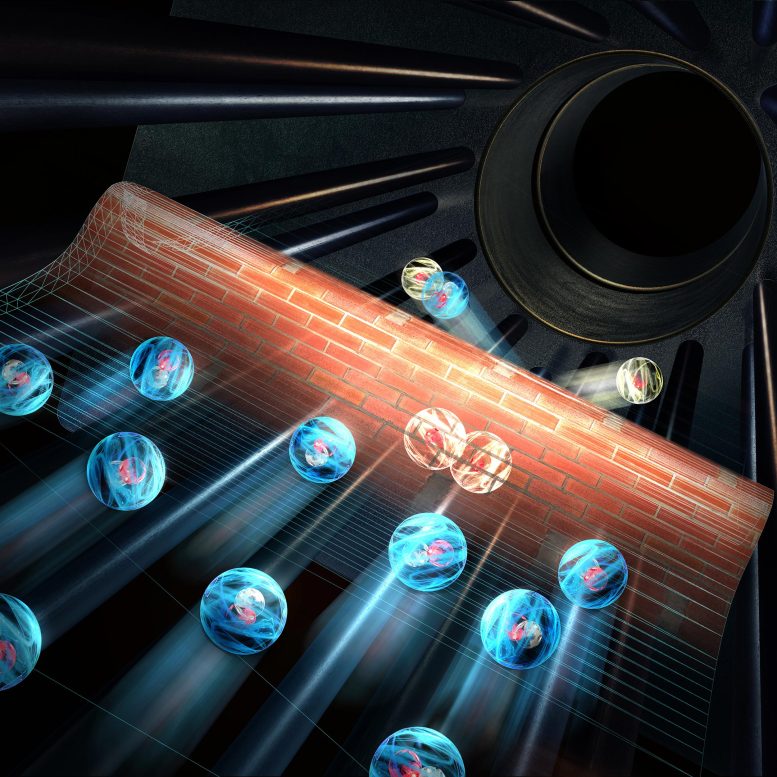
Quantum mechanics allows particles due to their quantum mechanical wave properties to break through the energetic barrier (wall) and a reaction occurs. Credit: Universität Innsbruck/Harald Ritsch
Breakthrough for modeling tunneling reactions in molecular chemistry.
Tunneling reactions in chemistry are very difficult to predict. The quantum mechanically exact description of chemical reactions with more than three particles is difficult, with more than four particles it is almost impossible. Theorists simulate these reactions with classical physics and must neglect quantum effects. But where is the limit of this classical description of chemical reactions, which can only provide approximations?
Roland Wester from the Department of Ion Physics and Applied Physics at the University of Innsbruck has long wanted to explore this frontier. “It requires an experiment that allows very precise measurements and can still be described quantum-mechanically,” says the experimental physicist. “The idea came to me 15 years ago in a conversation with a colleague at a conference in the U.S.,” Wester recalls. He wanted to trace the quantum mechanical tunnel effect in a very simple reaction.
Since the tunnel effect makes the reaction very unlikely and thus slow, its experimental observation was extraordinarily difficult. After several attempts, however, Wester’s team has now succeeded in doing just that for the first time, as they report in the current issue of the journal Nature.
Breakthrough after 15 years of research
Roland Wester’s team chose hydrogen – the simplest element in the universe – for their experiment. They introduced deuterium – a hydrogen isotope – into an ion trap, cooled it down and then filled the trap with hydrogen gas. Because of the very low temperatures, the negatively charged deuterium ions lack the energy to react with hydrogen molecules in the conventional way. In very rare cases, however, a reaction does occur when the two collide.
This is caused by the tunnel effect: “Quantum mechanics allows particles to break through the energetic barrier due to their quantum mechanical wave properties, and a reaction occurs,” explains the first author of the study, Robert Wild. “In our experiment, we give possible reactions in the trap about 15 minutes and then determine the amount of hydrogen ions formed. From their number, we can deduce how often a reaction has occurred.”
In 2018, theoretical physicists had calculated that in this system quantum tunneling occurs in only one in every hundred billion collisions. This corresponds very closely with the results now measured in Innsbruck and, after 15 years of research, for the first time confirms a precise theoretical model for the tunneling effect in a chemical reaction.
Foundation for a better understanding
There are other chemical reactions that might exploit the tunnel effect. For the first time, a measurement is now available that is also well-understood in scientific theory. Based on this, research can develop simpler theoretical models for chemical reactions and test them on the reaction that has now been successfully demonstrated.
The tunnel effect is used, for example, in the scanning tunneling microscope and in flash memories. The tunnel effect is also used to explain the alpha decay of atomic nuclei. By including the tunnel effect, some astrochemical syntheses of molecules in interstellar dark clouds can also be explained. The experiment of Wester’s team thus lays the foundation for a better understanding of many chemical reactions.
Reference: “Tunneling measured in a very slow ion-molecule reaction” by Robert Wild, Markus Nötzold, Malcolm Simpson, Thuy Dung Tran and Roland Wester, 1 March 2023, Nature.
DOI: 10.1038/s41586-023-05727-z
The research was financially supported by the Austrian Science Fund FWF and the European Union, among others.







How do the researchers distinguish this from the very low probability, but statistically possible events of some molecules gaining the required energy from normal collisions in the gas?
Interesting question from the first commenter.
My preference is to use a temperature sensitive “lock and key” analogy for tunneling here, especially as it has analogues in living processes. The most obvious variables fitting a “tunneling key” to a “tunneling lock” to me are in molecular spin and vibrational energies. Vibration in bonds allows, with low probability, all the bonds to be simultaneously expanded, or contracted, which seems most capable of affecting tunnel rates. Of potential interest to me is the possibility of critical points in tunneling rate with temperature variations and reaction chamber rotations.
Energy gain may be observed in between leaves as if gathered from thin leaf air and a possible quantum gain controlling the capacitance in between for a bonding.