
The study also shed light on how photosynthesis adapted to the rise of oxygen.
Back to the future of photosynthesis.
Rubisco, the central biocatalyst in photosynthesis, is the most prevalent enzyme on the planet. A group of Max Planck Institute researchers has uncovered one of the key early photosynthesis adaptations by reconstructing billion-year-old enzymes. Their findings not only shed light on how modern photosynthesis evolved, but also provide new impulses for enhancing it.
Today’s life is entirely dependent on photosynthetic organisms such as plants and algae that capture and convert CO2. An enzyme known as Rubisco, which absorbs more than 400 billion tons of CO2 annually, is at the heart of these processes. Rubisco is produced in astounding quantities by living things today; its mass on Earth exceeds that of all humans combined. Rubisco has to continually adapt to shifting environmental circumstances in order to play such a major role in the global carbon cycle.
A team from the Max Planck Institute for Terrestrial Microbiology in Marburg, Germany, in partnership with the University of Singapore, has now successfully resurrected and studied billion-year-old enzymes in the lab using a combination of computational and synthetic techniques. The researchers discovered that in this process, which they refer to as “molecular paleontology,” a completely new component prepared photosynthesis to adapt to increased oxygen levels rather than direct mutations in the active center.
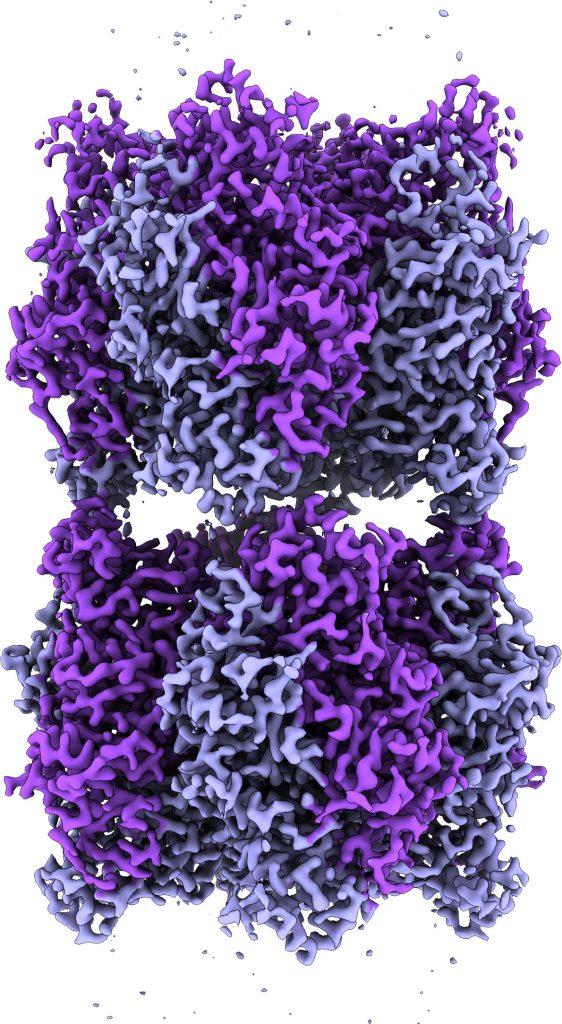
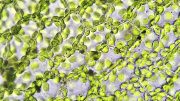
Cryo-electron microscope image of two Rubisco complexes interacting with each other. If a subunit essential for solubility is missing, individual enzyme complexes can interact with each other in this way and form thread-like structures, so-called fibrils. Under normal conditions, however, Rubisco does not form such fibrils. Credit: MPI f. Terrestrial Microbiology/ L. Schulz
Rubisco’s early confusion
Rubisco is ancient: it emerged approximately four billion years ago in primordial metabolism prior to the presence of oxygen on earth. However, with the invention of oxygen-producing photosynthesis and the rise of oxygen in the atmosphere, the enzyme started catalyzing an undesired reaction, in which it mistakes O2 for CO2 and produces metabolites that are toxic to the cell. This confused substrate scope still scars Rubiscos to date and limits photosynthetic efficiency. Even though Rubiscos that evolved in oxygen-containing environments became more specific for CO2 over time, none of them could get completely rid of the oxygen-capturing reaction.
The molecular determinants of increased CO2 specificity in Rubisco remain largely unknown. However, they are of great interest to researchers aiming to improve photosynthesis. Interestingly, those Rubiscos that show increased CO2 specificity recruited a novel protein component of unknown function. This component was suspected to be involved in increasing CO2 specificity, however, the true reason for its emergence remained difficult to determine because it already evolved billions of years ago.
Studying evolution by resurrecting ancient proteins in the lab
To understand this key event in the evolution of more specific Rubiscos, collaborators at the Max Planck Institute for Terrestrial Microbiology in Marburg and Nanyang Technological University in Singapore used a statistical algorithm to recreate forms of Rubiscos that existed billions of years ago, before oxygen levels began to rise. The team led by Max Planck researchers Tobias Erb and Georg Hochberg resurrected these ancient proteins in the lab to study their properties. In particular, the scientists wondered whether Rubisco’s new component had anything to do with the evolution of higher specificity.
The answer was surprising, as doctoral researcher Luca Schulz explains: “We expected the new component to somehow directly exclude oxygen from Rubisco catalytic center. That is not what happened. Instead, this new subunit seems to act as a modulator for evolution: recruitment of the subunit changed the effect that subsequent mutations had on Rubisco’s catalytic subunit. Previously inconsequential mutations suddenly had a huge effect on specificity when this new component was present. It seems that having this new subunit completely changed Rubisco’s evolutionary potential.”
An enzyme’s addiction to its new subunit
This function as an “evolutionary modulator” also explains another mysterious aspect of the new protein component: Rubiscos that incorporated it are completely dependent on it, even though other forms of Rubisco can function perfectly well without it. The same modulating effect explains why: When bound to this small protein component, Rubisco becomes tolerant to mutations that would otherwise be catastrophically detrimental. With the accumulation of such mutations, Rubisco effectively became addicted to its new subunit.
Altogether, the findings finally explain the reason why Rubisco kept this new protein component around ever since it encountered it. Max Planck Research Group Leader Georg Hochberg explains: “The fact that this connection was not understood until now highlights the importance of evolutionary analysis for understanding the biochemistry that drives life around us. The history of biomolecules like Rubisco can teach us so much about why they are the way they are today. And there are still so many biochemical phenomena whose evolutionary history we really have no idea about. So it’s a very exciting time to be an evolutionary biochemist: almost the entire molecular history of the cell is still waiting to be discovered.”
Scientific journeys back in time can provide invaluable insights for the future
The study also has important implications for how photosynthesis might be improved, says Max Planck Director Tobias Erb: “Our research taught us that traditional attempts to improve Rubisco might have been looking in the wrong place: for years, research focused solely on changing amino acids in Rubisco itself to improve it. Our work now suggests that adding entirely new protein components to the enzyme could be more productive and may open otherwise impossible evolutionary paths. This is uncharted land for enzyme engineering.”
Reference: “Evolution of increased complexity and specificity at the dawn of form I Rubiscos” by Luca Schulz, Zhijun Guo, Jan Zarzycki, Wieland Steinchen, Jan M. Schuller, Thomas Heimerl, Simone Prinz, Oliver Mueller-Cajar, Tobias J. Erb and Georg K. A. Hochberg, 13 October 2022, Science.
DOI: 10.1126/science.abq1416





It goes to show that we have a lot to learn about genes, enzymes, gut bacteria etc., mainly about genes. Since we have already deciphered Genomes of Mice & Rats, it is essential to transfer a gene in between them, record the result and start freezing their cadavers at Absolute temperatures for future generations to continue their work on them and also to prevent mingling with General Population. After continuing such study with other genes also, if and when an Eureka event arises on one fine day, that will be great !
We have learnt to make use of Solar, Wind, Hydro, Thermal and Nuclear energies. But, We still depend on Plants to clean up the air for us and provide oxygen. There must be a shortcut to copy all secrets of plants and start factories to do the same that they all do. Then, we may have a way to continue to use Oil & Natural Gas as before !
RUBISCOS: So, We go and dig for archaeological ornaments, but never cared to create a data of various past Rubiscos yet? We focus on old shipwrecks, which will still be there. They will not disappear. But, people of this generation will disappear in decades, which indicates our priorities are not set right. 200 years from now, they will dig for our items? Very Silly. Focus on Genetics & Biochemistry first to improve human lives !