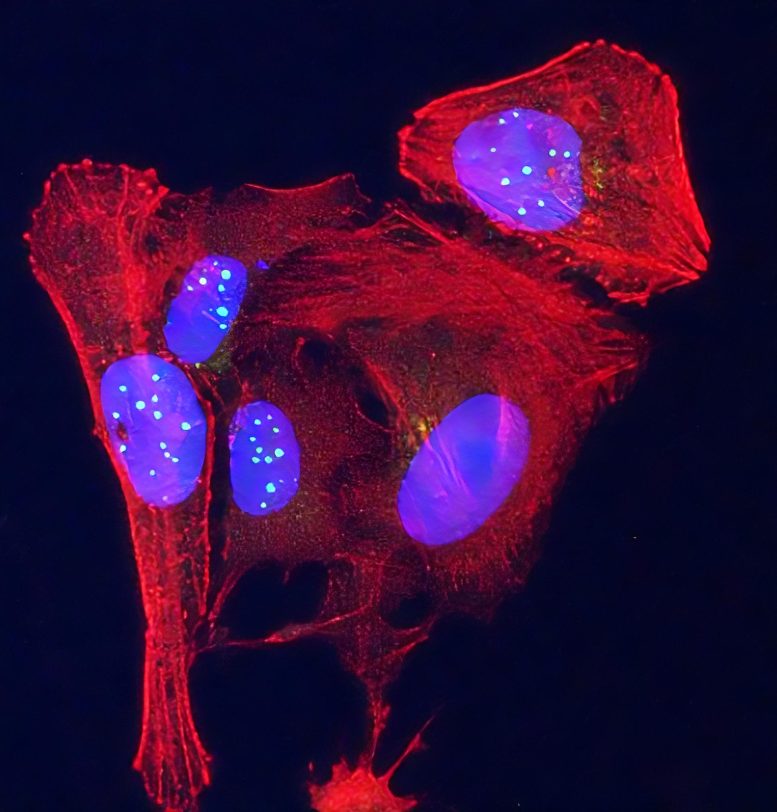
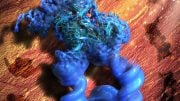
Newly discovered telomeric protein VR, (green spheres) is seen accumulating in nuclei (blue ovals) in human osteosarcoma cancer cells stained in red. Credit: Griffith Lab, UNC Lineberger
Researchers Jack Griffith and Taghreed Al-Turki from the UNC School of Medicine have discovered that telomeres, located at the ends of chromosomes, possess enough genetic information to produce two small proteins with potentially potent biological properties.
Telomeres, previously believed to be incapable of encoding proteins due to their repetitive DNA sequences, have now been found to hold a potent biological function that may have significant implications for our understanding of cancer and aging.
In a study published in the Proceedings of the National Academy of Science, Taghreed Al-Turki, Ph.D., and Jack Griffith, Ph.D., from the UNC School of Medicine, made a remarkable finding that telomeres contain genetic information to produce two small proteins. The researchers discovered that one of these proteins is elevated in some human cancer cells and cells from patients with telomere-related defects.
“Based on our research, we think simple blood tests for these proteins could provide a valuable screen for certain cancers and other human diseases,” said Griffith, the Kenan Distinguished Professor of Microbiology and Immunology and member of the UNC Lineberger Comprehensive Cancer Center. “These tests also could provide a measure of ‘telomere health,’ because we know telomeres shorten with age.”
Telomeres contain a unique DNA sequence consisting of endless repeats of TTAGGG bases that somehow inhibit chromosomes from sticking to each other. Two decades ago, the Griffith laboratory showed that the end of a telomere’s DNA loops back on itself to form a tiny circle, thus hiding the end and blocking chromosome-to-chromosome fusions. When cells divide, telomeres shorten, eventually becoming so short that the cell can no longer divide properly, leading to cell death.
Scientists first identified telomeres about 80 years ago, and because of their monotonous sequence, the established dogma in the field held that telomeres could not encode for any proteins, let alone ones with potent biological function.
In 2011 a group in Florida working on an inherited form of ALS reported that the culprit was an RNA molecule containing a six-base repeat which by a novel mechanism could generate a series of toxic proteins consisting of two amino acids repeating one after the other. Al-Turki and Griffith note in their paper a striking similarity of this RNA to the RNA generated from human telomeres, and they hypothesized that the same novel mechanism might be in play.
They conducted experiments – as described in the PNAS paper – to show how telomeric DNA can instruct the cell to produce signaling proteins they termed VR (valine-arginine) and GL (glycine-leucine). Signaling proteins are essentially chemicals that trigger a chain reaction of other proteins inside cells that then lead to a biological function important for health or disease.
Al-Turki and Griffith then chemically synthesized VR and GL to examine their properties using powerful electron and confocal microscopes along with state-of-the-art biological methods, revealing that the VR protein is present in elevated amounts in some human cancer cells, as well as cells from patients suffering from diseases resulting from defective telomeres.
“We think it’s possible that as we age, the amount of VR and GL in our blood will steadily rise, potentially providing a new biomarker for biological age as contrasted to chronological age,” said Al-Turki, a postdoctoral researcher in the Griffith lab. “We think inflammation may also trigger the production of these proteins.”
Griffith noted, “When you go against current thinking, you are usually wrong because you are bucking many people who’ve worked so diligently in their fields. But occasionally scientists have failed to put observations from two very distant fields together and that’s what we did. Discovering that telomeres encode two novel signaling proteins will change our understanding of cancer, aging, and how cells communicate with other cells.
“Many questions remain to be answered, but our biggest priority now is developing a simple blood test for these proteins. This could inform us of our biological age and also provide warnings of issues, such as cancer or inflammation.”
Reference: “Mammalian telomeric RNA (TERRA) can be translated to produce valine–arginine and glycine–leucine dipeptide repeat proteins” by Taghreed M. Al-Turki and Jack D. Griffith, 22 February 2023, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2221529120
The study was funded by the National Institutes of Health. The UNC Viral Vector Core and the light microscopy facility at the UNC Lineberger Comprehensive Cancer Center were vital partners in this research. Jack Griffith has a joint faculty appointment in the UNC Department of Biochemistry and Biophysics.


Be the first to comment on "Telomere Twist: Unraveling the Hidden Protein Secrets of Cancer and Aging"