
Scientists have found that verteporfin, a drug already approved by the FDA for eye disease, stopped the replication of SARS-CoV-2, the virus that causes COVID-19.
An interdisciplinary research team led by the University of California, Los Angeles (UCLA) discovered that a drug already approved by the Food and Drug Administration (FDA) for eye disease, verteporfin, stopped the replication of SARS-CoV-2, the virus that causes COVID-19. Their laboratory study identified the Hippo signaling pathway as a potential target for therapies against the coronavirus.
Background
Many important human biological processes are controlled by complicated chain reactions called signaling pathways, in which certain proteins act as messenger molecules that promote or block the signals of other proteins.
The lead researchers were investigating the Hippo pathway, which controls the size of organs in the body, in earlier National Institutes of Health–funded studies of the Zika virus, which can cause undersized brains in infants. Noticing that this pathway also seemed to have virus-fighting effects, they launched the current study investigating SARS-CoV-2.
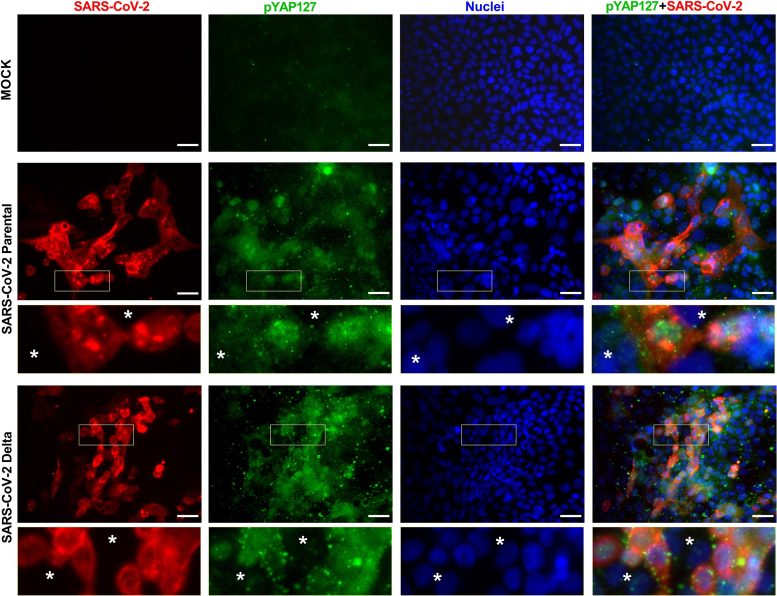
This chart shows levels of SARS-CoV-2 and deactivated YAP (pYAP127) in healthy cultured cells (mock) and cultured cells infected with the original strain of COVID-19 (SARS-CoV-2 Parental) and the Delta strain (SARS-CoV-2 Delta). Asterisks in the insets indicate uninfected cells. Credit: UCLA/Broad Stem Cell Research Center
Method
The scientists performed experiments using tissue samples from people with COVID-19, as well as cultured human heart and lung cells selected to closely reflect how healthy cells respond to SARS-CoV-2 infection. They observed changes in many genes involved with the Hippo signaling pathway after infection. In addition, they examined a protein called YAP, or Yes-associated protein, whose activity is blocked when the Hippo pathway is activated.
The scientists found that in the cultured human cells, both the original strain and Delta variant of SARS-CoV-2 activated the Hippo pathway in the first few days after infection. When they silenced this pathway and increased YAP, the virus replicated itself more. They team also pretreated cells with verteporfin, which blocks YAP in the eye disease known as choroidal neovascularization, and then infected them with SARS-CoV-2. In the verteporfin-treated cells, concentrations of the coronavirus were below detectable levels, compared to more than 60,000 units of the virus per milliliter in an untreated control group.
Impact
The results indicate verteporfin may be a candidate to treat COVID-19, and its status as FDA-approved could make it easier to launch clinical trials to verify its safety and effectiveness against the coronavirus. The study showed that the Hippo pathway is activated within days of SARS-CoV-2 infection, suggesting that treatments using the mechanism could be deployed before symptoms arise to reduce the severity of disease.
Reference: “Hippo signaling pathway activation during SARS-CoV-2 infection contributes to host antiviral response” by Gustavo Garcia Jr., Arjit Vijey Jeyachandran, Yijie Wang, Joseph Ignatius Irudayam, Sebastian Castillo Cario, Chandani Sen, Shen Li, Yunfeng Li, Ashok Kumar, Karin Nielsen-Saines, Samuel W. French, Priya S. Shah, Kouki Morizono, Brigitte N. Gomperts, Arjun Deb, Arunachalam Ramaiah, Vaithilingaraja Arumugaswami, 8 November 2022, PLOS Biology.
DOI: 10.1371/journal.pbio.3001851
The study’s first author is Gustavo Garcia Jr., a former UCLA staff research associate, and the corresponding authors are Vaithilingaraja Arumugaswami, a UCLA associate professor of molecular and medical pharmacology and a member of the California NanoSystems Institute at UCLA and the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA, and Arunachalam Ramaiah of the Tata Institute for Genetics and Society in India. Other co-authors are Arjit Jeyachandran, Yijie Wang, Joseph Irudayam, Sebastian Castillo Cario, Chandani Sen, Shen Li, Yunfeng Li, Karin Nielsen-Saines, Samuel French, Kouki Morizono, Brigitte Gomperts, and Arjun Deb, all of UCLA; Ashok Kumar of Wayne State University; and Priya Shah of UC Davis.
The study was funded by the UCLA David Geffen School of Medicine, the Broad Stem Cell Research Center, the UCLA W.M. Keck Foundation COVID-19 Research Award Program, the National Institutes of Health (NIH), and the Tata Institute.







Be the first to comment on "UCLA Scientists Say FDA-Approved Eye-Disease Drug May Also Help Fight COVID"