
Recent research identifies the latrophilin 1 receptor as a potential regulator of eating behavior, suggesting new approaches to treating obesity by targeting this receptor.
A new potential approach to treating obesity has been identified.
The rising prevalence of overweight and obesity is a significant global medical challenge. Alongside lifestyle changes, genetic factors play a crucial role in the development of obesity. Researchers at Leipzig University and Heinrich Heine University Düsseldorf have identified a new regulator of eating behavior. The findings have been published in the internationally renowned Nature journal Signal Transduction and Targeted Therapy.
“Our research underscores our incomplete understanding of the factors governing food intake. It also reveals the potential involvement of previously overlooked receptors,” says Dr Doreen Thor, lead author of the study and scientist at the Faculty of Medicine at Leipzig University. The newly identified receptor, latrophilin 1, has primarily been studied for its functions in the brain such as synapse formation and development, but not for its role in controlling food intake. It is a G protein-coupled receptor and was so named because of its ability to bind to the neurotoxin latrotoxin. This toxin is produced by some spiders, including the Mediterranean black widow, and has the latrophilin 1 receptor as a key neuronal target structure.
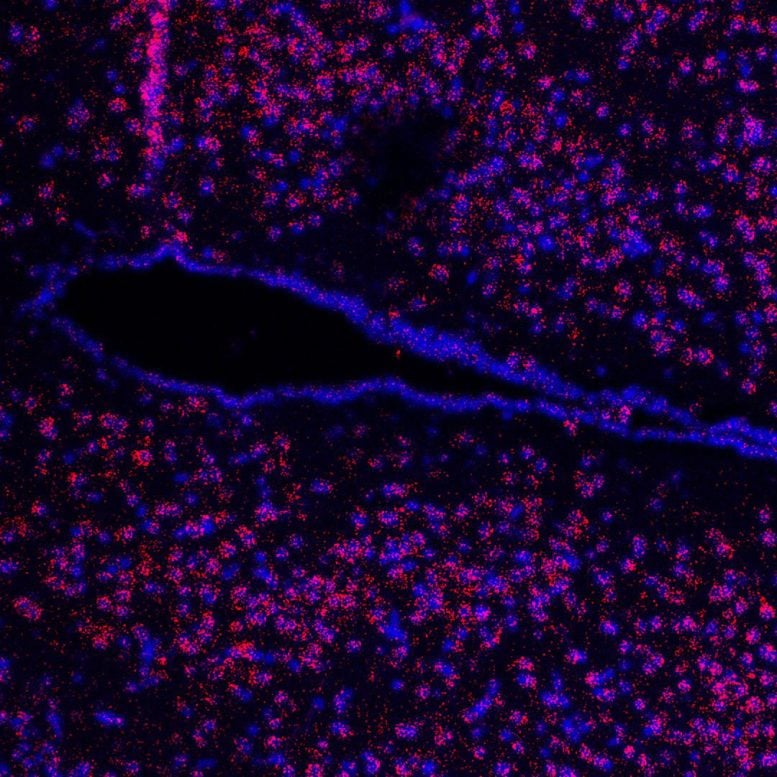
Microscopic view of a hypothalamus brain section. The third ventricle (black) is visible. The red dots show the receptor. The nuclei are blue. Credit: Leipzig University (Albert Ricken)
Impact on Eating Behavior and Physical Activity
In their studies, the research teams led by Dr. Thor from Leipzig University and Professor Simone Prömel from Heinrich Heine University Düsseldorf show that the latrophilin 1 receptor is present in the regions of the brain that control eating behaviour as well as in adipose tissue. In the study, mice lacking the receptor showed increased food intake and reduced physical activity. Although the juveniles were initially of normal weight, they became significantly overweight over the course of a further four months. This in turn leads to the well-known comorbidities of obesity, such as fatty liver and diabetes mellitus.
In addition, the researchers identified a receptor variant of latrophilin 1 in the sequencing data from the Leipzig obesity cohort that occurred in one patient with overweight. Cell culture studies indicated this variant’s impaired functionality, suggesting that the receptor may play a role in the development of obesity not only in animal models, but also in humans.
“The results provide a new approach to understanding the regulation of food intake and the development of obesity,” says Professor Simone Prömel, another corresponding author of the paper. Future studies in the participating research groups at the universities in Leipzig and Düsseldorf will now clarify whether the receptor can serve as a potential pharmacological target for the regulation of food intake in obesity.
Reference: “Dysfunction of the adhesion G protein-coupled receptor latrophilin 1 (ADGRL1/LPHN1) increases the risk of obesity” by André Nguyen Dietzsch, Hadi Al-Hasani, Joachim Altschmied, Katharina Bottermann, Jana Brendler, Judith Haendeler, Susanne Horn, Isabell Kaczmarek, Antje Körner, Kerstin Krause, Kathrin Landgraf, Diana Le Duc, Laura Lehmann, Stefan Lehr, Stephanie Pick, Albert Ricken, Rene Schnorr, Angela Schulz, Martina Strnadová, Akhil Velluva, Heba Zabri, Torsten Schöneberg, Doreen Thor and Simone Prömel, 26 April 2024, Signal Transduction and Targeted Therapy.
DOI: 10.1038/s41392-024-01810-7







Be the first to comment on "New Potential Obesity Treatment Discovered: Hidden Brain Receptor May Hold Key to Controlling Your Appetite"