Researchers have significantly improved the efficiency of photoclick chemistry. Using molecular substitutions, they enhanced the reactivity of the PQ-ERA reaction, leading to higher quantum yields and reaction rates.
Through a collaborative effort involving the Universities of Groningen and Amsterdam in the Netherlands, along with Italy’s European Laboratory for Non-Linear Spectroscopy, scientists have significantly advanced photoclick chemistry. They enhanced the reactivity of the photoclick compound used in the widely-utilized PQ-ERA reaction through strategic molecular substitution.
In Chemical Science, the flagship journal of the Royal Society of Chemistry, they report a superb photoreaction quantum yield, high reaction rates, and notable oxygen tolerance. The paper was designated a HOT Article as well as Pick of the Week.
At the University of Amsterdam, Michiel Hilbers and Wybren Jan Buma of the Molecular Photonics group (Van ’t Hoff Institute for Molecular Sciences) contributed to the research of which the synthetic and reaction characterization part was conducted in the laboratories of Wiktor Szymanski and Nobel laureate Ben Feringa at the University of Groningen.
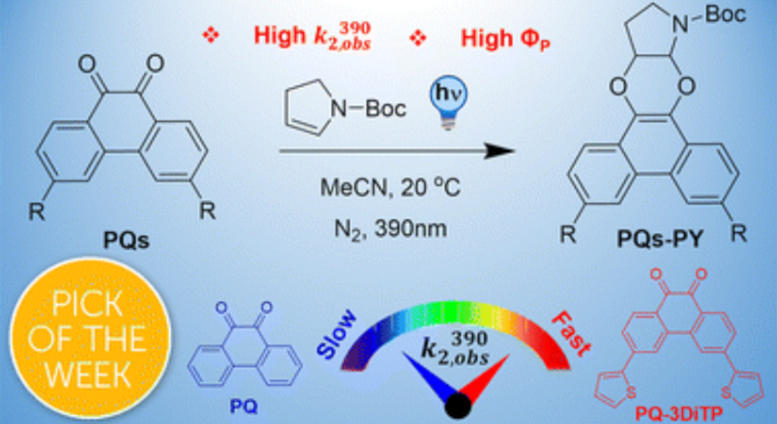
Graphical abstract of the research. Credit: UvA HIMS / ChemSci
Photoclick chemistry is a light-activated variety of click chemistry (Nobel Prize in Chemistry 2022), a set of elegant and efficient chemical reaction methods that couple dedicated molecular units to yield desired products. Photoclick chemistry has unique advantages over conventional click chemistry as it allows for a high degree of both spatial and temporal control over the reaction. It has a broad range of applications, including 3D printing, protein labeling, and bioimaging.
A boost for the PQ-ERA photoclick reaction
A specific photoclick reaction is the so-called PQ-ERA reaction – the light-induced photocycloaddition of 9,10-phenanthrenequinone (PQ) with electron-rich alkenes (ERA). It has drawn much attention because of its excellent kinetics and biocompatibility. However, the conventionally used PQ compounds show limited reactivity, which hinders its overall efficiency.
In the study now presented in Chemical Science, the international research team presents a simple strategy to change that. They describe how a thiophene substitution at the 3-position of the PQ scaffold significantly boosts the reactivity of the PQ triplet state to enhance the efficiency of the PQ-ERA reaction.
Nanosecond time-resolved spectroscopic studies and quantum chemical studies in the Amsterdam Molecular Photonics group combined with femtosecond time-resolved spectroscopic studies performed in Florence provided a fundamental understanding of this specific photoclick chemistry. The investigations show that the substitution significantly increases the population of the reactive triplet state (3ππ*) during excitation of 3-thiophene PQs. This results in a superb photoreaction quantum yield (FP, up to 98%), high second-order rate constants (k2, up to 1974 M−1 s−1), and notable oxygen tolerance for the PQ-ERA reaction system.
These results now pave the way for a further improvement of the reaction, offering excellent prospects for fast and efficient photoclick transformations.
Reference: “Establishing PQ-ERA photoclick reactions with unprecedented efficiency by engineering of the nature of the phenanthraquinone triplet state” by Youxin Fu, Georgios Alachouzos, Nadja A. Simeth, Mariangela Di Donato, Michiel F. Hilbers, Wybren Jan Buma, Wiktor Szymanski and Ben L. Feringa, 21 June 2023, Chemical Science.
DOI: 10.1039/D3SC01760E







Be the first to comment on "Chemistry Breakthrough: Scientists Take Photoclick Chemistry to the Next Level"