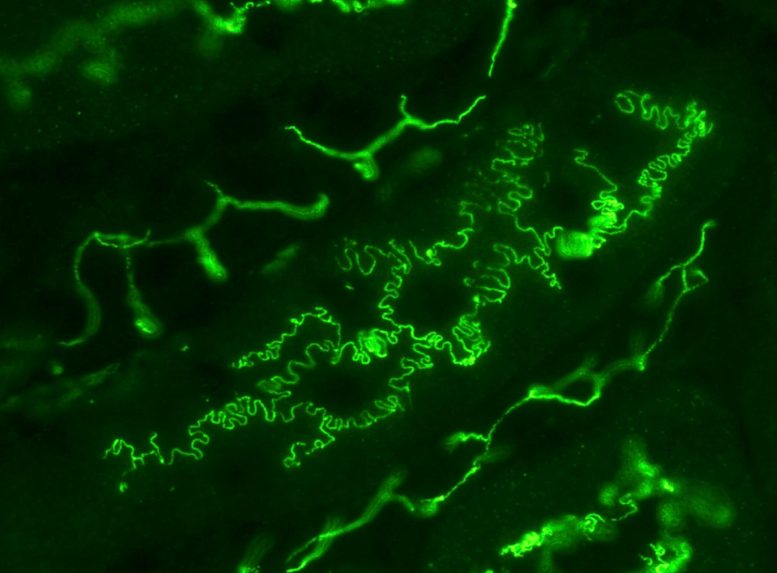
Photo of mouse renal tubules in vivo, immunostained for plasma membrane protein occludin (green). Many epithelial cells like the ones shown here have rugged boundaries described as interdigitated. It is proposed that interdigitation facilitates the transport of molecules and fluid between cell boundaries. Credit: Kyushu University/Miura Lab
The Mathematics of Cell Boundary “Ruggedness”
Researchers have uncovered both the mathematical and biological mechanism behind the rugged structures at cell boundaries found in tissues such as the kidneys and nasal glands. The team hopes that their new insights can help develop new ways of treating associated pathologies and build better biological models for future study.
Our cells come in all sorts of shapes and sizes. From the neurons that extend across the central nervous system, to the spherical white blood cells that protect us from infection, a cell’s form and structure are critical to its function in our body. The structures between cells can also vary, and similarly hold crucial utility.
One such in-between structure is the ‘rugged’ or ‘wavey’ pattern commonly found between epithelial cells, the type of cell that covers your skin and most other organs and blood vessels. Under a microscope, these patterns can look quite unruly, but to Professor Takashi Miura of Kyushu University’s Faculty of Medical Sciences, it is a subject of fascination.
“It almost looks like the interlocking teeth on a zipper. Researchers also describe these structures as interdigitated cell boundaries,” explains Miura who led the study published today (April 21) in iScience. “Many cells have interdigitated cell boundaries. For example, kidney podocytes that work as filters to generate urine have very intricate interdigitation patterns. Plant leaf epidermal cells look like a jigsaw puzzle so as to reduce the mechanical stress on cell walls.”
One critical function of epithelial cells is to facilitate the transport of molecules and fluid between said cell boundaries, a process known as paracellular transport. Recent work has proposed that interdigitation of the boundary enhances transport efficiency. However, how exactly these structures form—and its physiological significance—are still not fully understood.
“We began by studying the interdigitation in MDCK cells, a type of epithelial cell originally from kidneys and commonly used in studying epithelial pattern formation,” states Miura. “We found something unexpected when we mathematically broke down the cell-cell boundary pattern. It turns out that these seemingly random structures are not random at all, and in fact, are mathematically scaling. In other words, the pattern has self-similarity—if you magnified the boundary, it holds the same characteristics as the original pattern”
The team then explored established mathematical models to understand how and why the interdigitation patterns have this distinctive form. After a number of working hypotheses, they landed on a model called the Edwards-Wilkinson model.
“The Edwards-Wilkinson model is used to mathematically simulate a randomly shaking boundary with a function of minimizing the length of that boundary. The scaling of cell boundaries we found fits into this model,” continues Miura. “After this, our next step was to find the molecular mechanism responsible for these dynamics.”
The team focused on the role of actomyosin, the actin-myosin protein complex responsible for almost everything that requires force in cellular activities. When observing closely, they identified specific myosin proteins that would localize on the bending cell boundary.
Miura explains that their new findings give them a better understanding of the fundamentals of cell dynamics, and contribute to the larger trend of developing the mathematical underpinnings of biology.
“Mathematics has always been inextricably linked in the fields of Chemistry and Physics. Mathematically breaking down fundamental processes in Biology is still a relatively new domain that has grown markedly over the last 20 years,” he concludes. “I think it shows that the field of Biology is maturing. As we develop this field, it will give us new perspectives on the fundamentals of life, and the beauty of biological patterns.”
Reference: “Mechanism of interdigitation formation at apical boundary of MDCK cell” by Shintaro Miyazaki, Tetsuhisa Otani, Kei Sugihara, Toshihiko Fujimori, Mikio Furuse and Takashi Miura, 21 April 2023, iScience.
DOI: 10.1016/j.isci.2023.106594
Funding: Japan Society for the Promotion of Science



Be the first to comment on "Cracking the Code: The Math Behind Nature’s Cellular Puzzles"