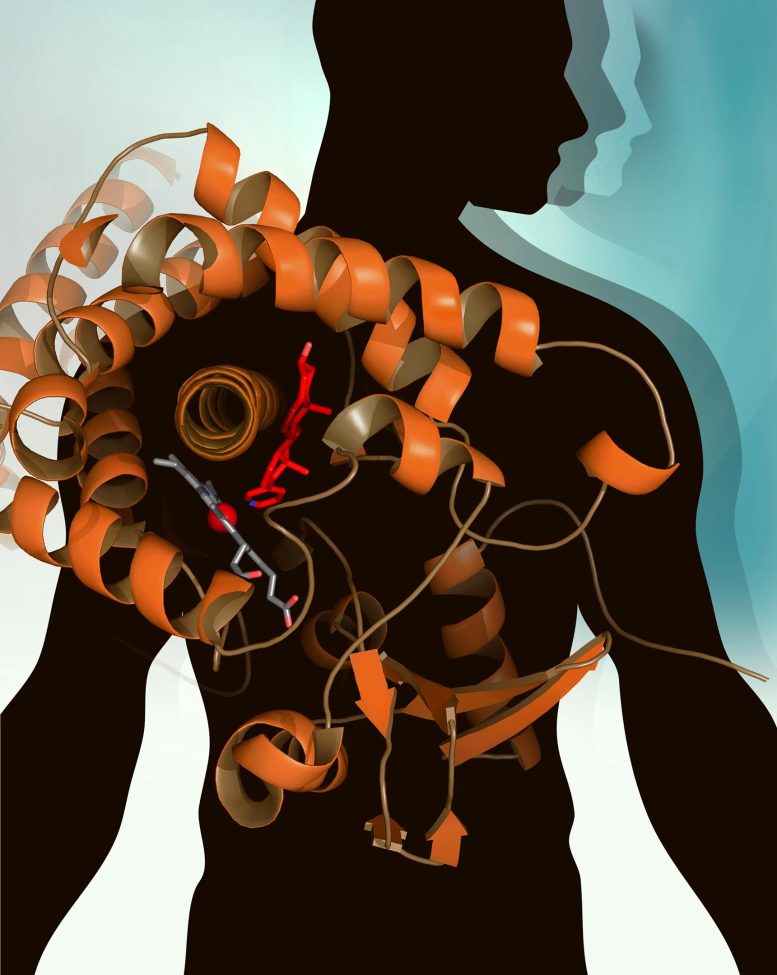
Scientists’ first glimpse of the structure of the human cytochrome P450 17A1 (blue ribbons) identified key structural and chemical features that are expected to facilitate drug design efforts. Credit: Image by Emily Scott, et al.
Using a modified version of Cytochrome P450 17A1, scientists from the University of Kansas have solved the structure of CYP17A1 for the first time, which should help enable the design of more effective drugs and improved treatments for prostate and breast cancer patients.
Prostate cancer, the most common cancer in men, is often a localized, slow-growing cancer, which aids treatment and improves survival rates. However, highly aggressive, metastatic forms of the cancer occur frequently enough to make it the No. 2 cause of death in U.S. men. Part of the reason for this high mortality rate is the lack of effective drugs to fight these more aggressive cancers.
SSRL is helping investigators change that.
Since prostate cancer cells proliferate in the presence of androgens – male hormones, such as testosterone – blocking the synthesis of androgens is an obvious strategy for treating prostate cancer. Researchers had even found a promising target in Cytochrome P450 17A1 (CYP17A1), an enzyme that produces androgens via a two-step process. But developing drugs to block CYP17A1 has proven challenging because of a lack of information about the enzyme’s structure. CYP17A1 is partially embedded in membranes, and the technical difficulties of forming it into crystals – the first step in determining its structure – had long frustrated scientists.
Emily Scott and Natasha DeVore from the University of Kansas solved that problem by turning E. coli bacteria into efficient factories for making a slightly modified version of CYP17A1, and then stabilizing this protein to form the necessary crystals. They brought the crystals to SSRL, where they had been granted rapid access approval to use Beam Line 9-2 to solve the structure of CYP17A1 for the first time.
The resulting new information has suggested ways to improve the one CYP17A1-inhibiting drug that is now available for treating metastatic prostate cancer. Approved in 2011, the active form of this drug, abiraterone, was designed using a combination of knowledge about the molecules that CYP17A1 uses to make androgens and trial-and-error chemistry approaches, but without the detailed knowledge provided by the structure of the enzyme.
One drawback of abiraterone is that it blocks both steps in the two-step process by which CYP17A1 produces androgens. The first reaction in that process is necessary to make hormones that control blood pressure and inflammation; thus patients who take abiraterone usually require other drugs to control high blood pressure, edema and cardiovascular side effects.
The new structural information provides an important starting point for ongoing studies at the University of Kansas and SSRL to determine how the two different reactions are controlled. This will facilitate the design of new drugs that halt only androgen production, and provide new avenues for prostate cancer patients.
Reference: “Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001” by Natasha M. DeVore and Emily E. Scott, 22 January 2012, Nature.
DOI: 10.1038/nature10743








Be the first to comment on "Data on CYP17A1 Structure May Lead to Advances in Prostate Cancer Drug Design"