
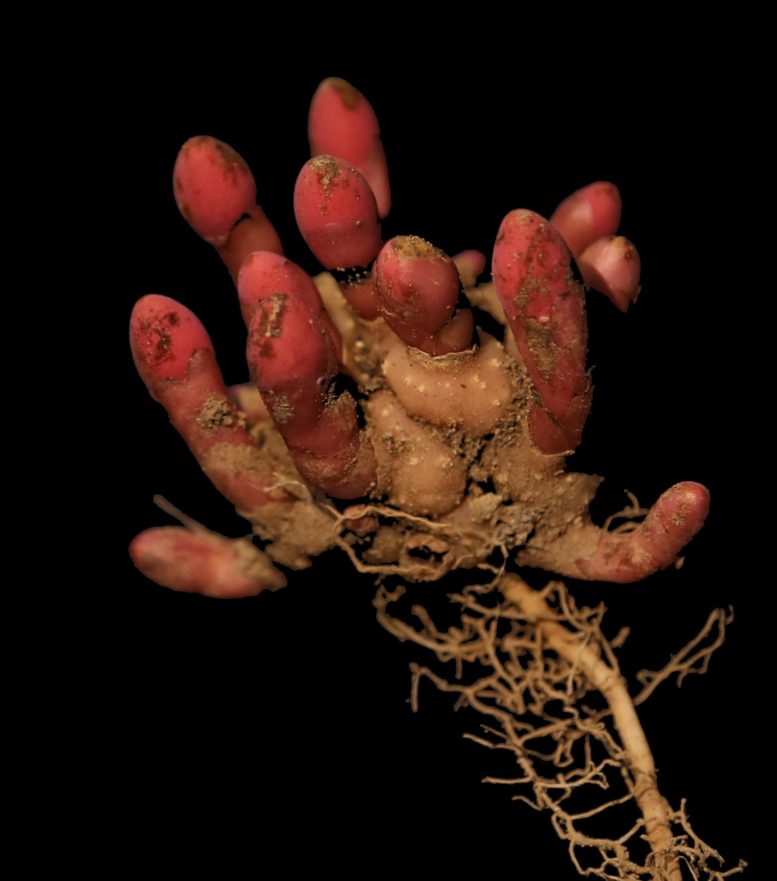
Balanophora shed one-third of its genes as it evolved into an uber-streamlined parasitic plant, according to new research by a team led by scientists at BGI Research, and including botanists from the University of British Columbia. Credit: Ze Wei, Plant Photo Bank of China, Nature Plants
If you happen to come across plants of the Balanophoraceae family in a corner of a forest, you might easily mistake them for fungi growing around tree roots. Their mushroom-like structures are actually inflorescences, composed of minute flowers.
But unlike some other parasitic plants that extend a haustorium into host tissue to steal nutrients, Balanophora induces the vascular system of their host plant to grow into a tuber, forming a unique underground organ with mixed host-parasite tissue. This chimeric tuber is the interface where Balanophora steals nutrients from its host plant.
Study on Extreme Parasitic Plants
How these subtropical extreme parasitic plants evolved into their current form piqued the interest of Dr. Xiaoli Chen. Dr. Chen is a scientist with BGI Research and the lead author of a new study published recently in the journal Nature Plants.
Dr. Chen and colleagues—including University of British Columbia botanist Dr. Sean Graham—compared the genomes of Balanophora and Sapria, another extreme parasitic plant in the family Rafflesiaceae that has a very different vegetative body.
The study revealed Sapria and Balanophora have lost 38 percent and 28 percent of their genomes respectively, while evolving to become holoparasitic—record shrinkages for flowering plants.
Balanophora shed one third of its genes as it evolved into an uber-streamlined parasitic plant, according to new research by a team led by scientists at BGI Research, and including botanists from the University of British Columbia. Credit: Xiaoli Chen, BGI Research, Nature Plants
Gene Losses and Convergent Evolution
“The extent of similar, but independent gene losses observed in Balanophora and Sapria is striking,” said Dr. Chen. “It points to a very strong convergence in the genetic evolution of holoparasitic lineages, despite their outwardly distinct life histories and appearances, and despite their having evolved from different groups of photosynthetic plants.”
The researchers found a near-total loss of genes associated with photosynthesis in both Balanophora and Sapria, as would be expected with the loss of photosynthestic capability.
However, the study also revealed a loss of genes involved in other key biological processes—root development, nitrogen absorption, and regulation of flowering development. The parasites have shed or compacted a large fraction of the gene families normally found in green plants—the large sets of duplicated gene plants that tend to perform related biological functions. This supports the idea that the parasites retain only those genes or gene copies that are essential.
Most astonishingly, genes related to the synthesis of a major plant hormone, abscisic acid (ABA), which is responsible for plant stress responses and signaling, have been lost in parallel in Balanophora and Sapria. Despite this, the researchers still recorded accumulation of the ABA hormone in flowering stems of Balanophora, and found that genes involved in the response to ABA signaling are still retained in the parasites.
Insights and Future Directions
“The majority of the lost genes in Balanophora are probably related to functions essential in green plants, which have become functionally unnecessary in the parasites,” said Dr. Graham.
“That said, there are probably instances where the gene loss was actually beneficial, rather than reflecting a simple loss of function. The loss of their entire ABA biosynthesis pathway may be a good example. It may help them to maintain physiological synchronization with the host plants. This needs to be tested in the future.”
Dr. Huan Liu, a researcher at BGI Research, emphasized the significance of the study in the context of 10KP—a project to sequence the genomes of 10,000 plant species.
“The study of parasitic plants deepens our understanding of dramatic genomic alterations and the complex interactions between parasitic plants and their hosts. The genomic data provides valuable insights into the evolution and genetic mechanisms behind the dependency of parasitic plants on their hosts, and how they manipulate host plants to survive.”
Reference: “Balanophora genomes display massively convergent evolution with other extreme holoparasites and provide novel insights into parasite–host interactions” by Xiaoli Chen, Dongming Fang, Yuxing Xu, Kunyu Duan, Satoko Yoshida, Shuai Yang, Sunil Kumar Sahu, Hui Fu, Xuanmin Guang, Min Liu, Chenyu Wu, Yang Liu, Weixue Mu, Yewen Chen, Yannan Fan, Fang Wang, Shufeng Peng, Dishen Shi, Yayu Wang, Runxian Yu, Wen Zhang, Yuqing Bai, Zhong-Jian Liu, Qiaoshun Yan, Xin Liu, Xun Xu, Huanming Yang, Jianqiang Wu, Sean W. Graham and Huan Liu, 21 September 2023, Nature Plants.
DOI: 10.1038/s41477-023-01517-7






Be the first to comment on "Extreme Genome Shrinkage: This Parasitic Plant Convinces Hosts To Grow Into Its Own Flesh"