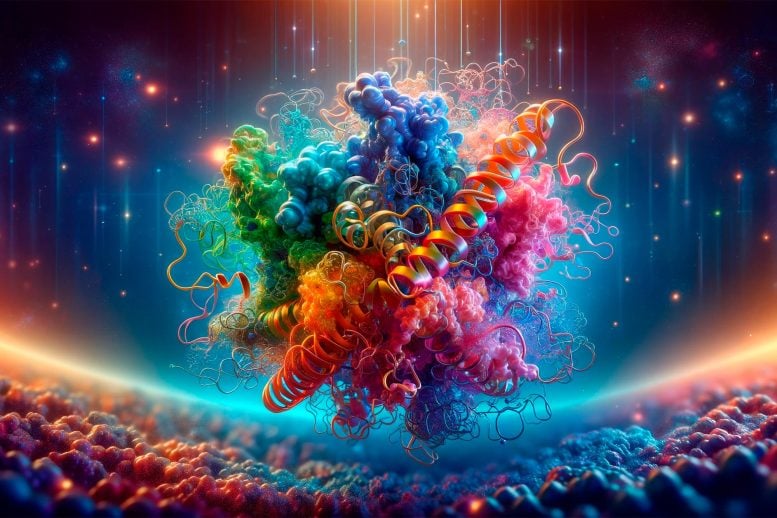
Researchers have found that studying proteins at body temperature reveals new drug binding sites, potentially revolutionizing drug design by providing more physiologically relevant insights into protein structure. Credit: SciTechDaily.com
Scientists discovered that proteins change shape at body temperature, revealing new drug binding sites. This finding, using cryo-electron microscopy, could revolutionize drug development by providing more accurate protein structures for medication design.
Some proteins change their shape in response to varying temperatures, unveiling previously hidden binding sites for drugs.
The findings, published in Nature, could revolutionize wide swathes of biology by fundamentally changing how protein structure is studied and leveraged for drug design. Van Andel Institute’s Juan Du, Ph.D. and Wei Lü, Ph.D led the research.
Proteins generally are investigated at low temperatures to ensure their stability. However, the new study demonstrates that certain proteins are highly sensitive to temperature and change their shape when viewed at body temperature.
“For a long time, the methods we’ve used to study proteins require them to be cold or frozen. But in the real world, human proteins exist and function at body temperature,” Du said. “Our study describes a new way to study proteins at body temperature and reveals that some proteins drastically alter their structures when warm, opening up new opportunities for structure-guided drug development.”
The Role of Proteins in Medication Development
Proteins are the molecular workhorses of the body. Their shape governs how they interact with other molecules to do their jobs. By determining protein structure, scientists can create blueprints that guide the development of more effective medications, much like locksmiths designing keys to fit into specific locks.
Although it is well known that temperature affects molecular function in the body, studying proteins at physiological temperature has been technologically challenging. Today’s study by the Du and Lü laboratories details how they overcame these issues and provides scientists a roadmap for doing so in their own experiments.
Proteins usually are studied at low temperatures. However, new findings from the labs of Dr. Juan Du and Dr. Wei Lu at Van Andel Institute demonstrate that certain proteins, such as TRPM4, are highly sensitive to temperature and change their shape when viewed at body temperature. This change may expose previously hidden binding sites for medications. Credit: Courtesy of the Du Lab and Lü Lab, Van Andel Institute
The study focused on a protein called TRPM4, which supports heart function and metabolism, including the release of insulin. As such, TRPM4 is linked to stroke, heart disease, and diabetes, among other health conditions.
To visualize TRPM4 at body temperature, the team leveraged VAI’s powerful suite of cryo-electron microscopes (cryo-EM), which allow scientists to flash freeze proteins and assemble detailed images of their structures. Rather than using a low-temperature sample, Postdoctoral Fellow Jinhong Hu, Ph.D., and colleagues in the Du and Lü laboratories heated the sample to body temperature before flash freezing it. By doing so, they found that ligands — molecules that bind to proteins — interact with totally different sites on TRPM4 at body temperature than at lower temperatures.
The implications of today’s study are far-reaching and reinforce the importance of studying proteins at body temperature to ensure the identification of physiologically relevant drug-binding sites.
Reference: “Physiological temperature drives TRPM4 ligand recognition and gating” by Jinhong Hu, Sung Jin Park, Tyler Walter, Ian J. Orozco, Garrett O‘Dea, Xinyu Ye, Juan Du and Wei Lü, 15 May 2024, Nature.
DOI: 10.1038/s41586-024-07436-7
The cryo-EM data was collected at VAI’s Cryo-EM Core and David Van Andel Cryo-Electron Microscopy Suite.
Research reported in this publication was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under award no. R01HL153219 (Lü); the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under awards no. R01NS112363 (Lü), R01NS111031 (Du) and R01NS129804 (Du); a Klingenstein-Simons Fellowship Award in Neuroscience (Du); an Alfred P. Sloan Research Fellowship in Neuroscience (Du); a Pew Scholar in Biomedical Sciences from the Pew Charitable Trusts (Du); a McKnight Scholar Award (Du); and the American Heart Association under award no. 24POST1196982 (Hu).







Be the first to comment on "Far-Reaching Implications – Scientists Discover Previously Hidden Binding Sites for Medications"