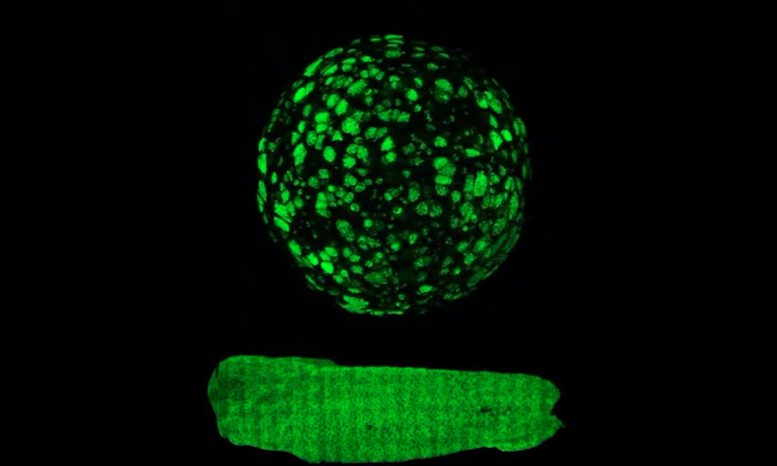
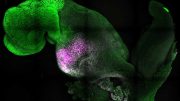
Green Slime covers the surface of a tadpole (bottom) and a goblet-cell regenerated aggregate (top, not the same scale). The images show the molecule intelectin-1, an important factor in tadpole skin, and one of the slime factors synthesized and secreted by goblet cells (single goblet cells can be seen in the aggregate). In the human lung, intelectin-1 binds bacteria and is on the front line of the innate immune system. Credit: Hye Young Kim and Lance Davidson
Lance Davidson of the University of Pittsburgh published an article in Nature Communications detailing that tissue mechanics can drive the regeneration of goblet cells on the outside surface of frog embryonic organoids.
Let’s talk about slime.
Mucus is a protective, slimy secretion produced by goblet cells and which lines organs of the respiratory, digestive, and reproductive systems. Slime production is essential to health, and an imbalance can be life-threatening. Patients with diseases such as asthma, chronic obstructive pulmonary disease (COPD), and ulcerative colitis produce too much mucus, often after growing too many goblet cells. Loss of goblet cells can be equally devastating — for instance during cancer, after infection, or injury. The balance of slime creation, amount, and transport is critical, so doctors and medical researchers have long sought the origins of goblet cells and have been eager to control processes that regenerate them and maintain balanced populations.
Recently, a group of bioengineers at the University of Pittsburgh discovered a case of goblet cell regeneration that is both easily accessible and happens incredibly fast on cells isolated from early-developing frog embryos. Their findings were published this week in the journal Nature Communications.
Lance Davidson, William Kepler Whiteford Professor of Bioengineering at Pitt, leads the MechMorpho Lab in the Swanson School of Engineering where his researchers study the role of mechanics in human cells as well as the Xenopus embryo — an aquatic frog native to South Africa.
“The Xenopus tadpole, like many frogs, has a respiratory skin that can exchange oxygen and perform tasks similar to a human lung,” explained Davidson. “Like the human lung, the surface of the Xenopus respiratory skin is a mucociliated epithelium, which is a tissue formed from goblet cells and ciliated cells that also protects the larva against pathogens. Because of these evolutionary similarities, our group uses frog embryonic organoids to examine how tissue mechanics impact cell growth and tissue formation.”
Studying this species is a rapid and cost-effective way to explore the genetic origins of biomechanics and how mechanical cues are sensed, not just in the frog embryo, but universally. When clinicians study cancer in patients, such changes can take weeks, months, or even years, but in a frog embryo, changes happen within hours.
“In this project, we took a group of mesenchymal cells out of the early embryo and formed them into a spherical aggregate, and within five hours, they began to change,” Davidson said. “These cells are known to differentiate into a variety of types, but in this scenario, we discovered that they changed very dramatically into a type of cell that they would not have changed into had they been in the embryo.”
The lab surprisingly uncovered a case of regeneration that restores a mucociliated epithelium from mesenchymal cells. They performed the experiment multiple times to confirm the unexpected findings and began to look closely at what microenvironmental cues could drive cells into an entirely new type.
“We have tools to modulate the mechanical microenvironment that houses the cells, and to our surprise, we found that if we made the environment stiffer, the aggregates changed into these epithelial cells,” explained Davidson. “If we made it softer, we were able to block them from changing. This finding shows that mechanics alone can cause important changes in the cells, and that is a remarkable thing.”
Davidson’s group is interested in how cells, influenced by mechanics, may affect disease states. The results detailed in this article may drive new questions in cancer biology, prompting researchers to consider whether certain kinds of invasive cancer cells might revert to a resting cell type based on the stiffness or softness of their surroundings.
“When applying these results to cancer biology, one might ask, ‘If tumors are surrounded by soft tissues, would they become dormant and basically non-invasive?’ Or, ‘If you have them in stiff tissues, would they invade and become deadly?'” said Davidson. “These are major questions in the field that biomechanics may be able to help answer. Many researchers focus solely on the chemical pathways, but we are also finding mechanical influencers of disease.”
Hye Young Kim, research fellow at the Korea Advanced Institute of Science and Technology (KAIST) and former member of the MechMorpho Lab, will continue this work at the Center for Vascular Research at KAIST’s Institute for Basic Science. She will study how cell motility changes during regeneration and how epithelial cells assemble a new epithelium. Davidson and his lab will explore how this novel case of mechanical cues is sensed by mesenchymal cells and how these mechanical induction pathways are integrated with known pathways that control cell fate choices.
“Frog embryos and organoids give us unparalleled access to study these processes, far more access than is possible with human organs,” he said. “The old ideas that regeneration is controlled exclusively by diffusing growth factors and hormones are giving way to the recognition that the physical mechanics of the environment — such as how rubbery or fluid the environment is — play just as critical a role.”
Reference: “Tissue mechanics drives regeneration of a mucociliated epidermis on the surface of Xenopus embryonic aggregates” by Hye Young Kim, Timothy R. Jackson, Carsten Stuckenholz and Lance A. Davidson, 31 January 2020, Nature Communications.
DOI: 10.1038/s41467-020-14385-y
This research was supported by a grant from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health.





Be the first to comment on "Got Slime? Regenerative Biology Used to Restore Mucus Production"