Researchers have elucidated the process by which an activated catalyst can break down the strong carbon-hydrogen (C-H) bonds of alkanes such as methane, a potent greenhouse gas. The study reveals the catalyst becomes active by a short flash of light, proceeding to break down the C-H bonds almost without any energy. Utilizing powerful X-ray light sources, SwissFEL and Swiss Light Source, the researchers followed the process from start to finish, witnessing the intricate exchange of electrons between the catalyst and the C-H group. Advanced quantum-chemical calculations helped interpret this complex process, potentially leading to better catalysts for the chemical industry, which could turn harmful methane and other alkanes into useful chemicals.
Scientists have deciphered how an activated catalyst breaks down the strong carbon-hydrogen bonds in potent greenhouse gas methane, according to a study published in Science. Using advanced X-ray technology and quantum-chemical calculations, they tracked the electron exchange between the catalyst and the methane molecule, paving the way for developing more efficient catalysts to convert harmful gases into useful chemicals.
The use of short flashes of X-ray light brings scientists one big step closer to developing better catalysts to transform the greenhouse gas methane into a less harmful chemical. The result, published in the journal Science, reveals for the first time how carbon-hydrogen bonds of alkanes break and how the catalyst works in this reaction.
Methane, one of the most potent greenhouse gases, is being released into the atmosphere at an increasing rate by livestock farming as well as the continuing unfreezing of permafrost. Transforming methane and longer-chain alkanes into less harmful and in fact useful chemicals would remove the associated threats, and in turn make a huge feedstock for the chemical industry available. However, transforming methane necessitates as a first step the breaking of a C-H bond, one of the strongest chemical linkages in nature.
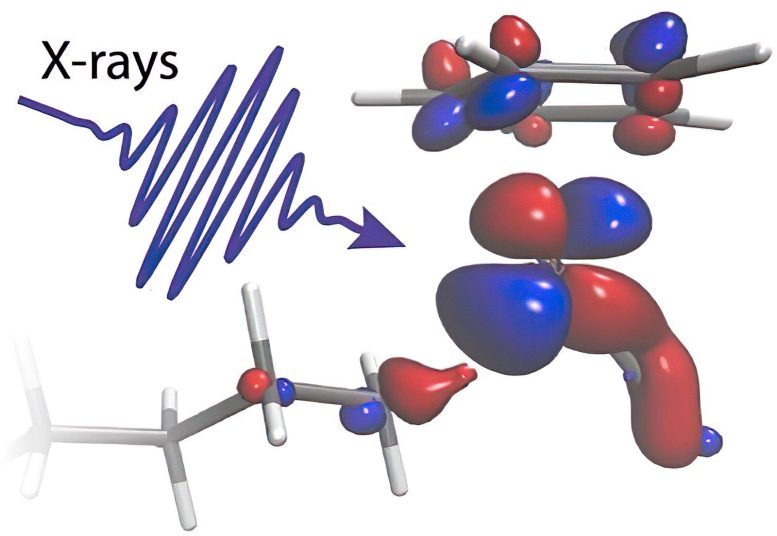
An X-ray flash illuminates a molecule. Credit: Raphael Jay
Forty years ago, molecular metal catalysts were discovered that can easily split C-H bonds. The only thing found to be necessary was a short flash of visible light to “switch on” the catalyst and, as by magic, the strong C-H bonds of alkanes passing nearby are easily broken almost without using any energy. Despite the importance of this so-called C-H activation reaction, it remained unknown over the decades how that catalyst performs this function.
The research was led by scientists from Uppsala University in collaboration with the Paul Scherrer Institute in Switzerland, Stockholm University, Hamburg University and the European XFEL in Germany. For the first time, the scientists were able to directly watch the catalyst at work and reveal how it breaks those C-H bonds.

Raphael Jay, Researcher at the Department of Physics and Astronomy, Uppsala University. Credit: Mikael Wallerstedt
In two experiments conducted at the Paul Scherrer Institute in Switzerland, the researchers were able to follow the delicate exchange of electrons between a rhodium catalyst and an octane C-H group as it gets broken. Using two of the most powerful sources of X-ray flashes in the world, the X-ray laser SwissFEL and the X-ray synchrotron Swiss Light Source, the reaction could be followed all the way from the beginning to the end. The measurements revealed the initial light-induced activation of the catalyst within 400 femtoseconds (0.0000000000004 seconds) to the final C-H bond breaking after 14 nanoseconds (0.000000014 seconds).
“The time-resolved X-ray absorption experiments we performed are only possible at large-scale facilities like SwissFEL and the Swiss Light Source, which provide extremely bright and short X-ray pulses. The catalyst is immersed in a dense octane solution, but by taking the perspective of the metal, we could specifically pick the one C-H bond out of hundreds of thousands which is made to break,” explains Raphael Jay, Researcher at Uppsala University and lead experimentalist of the study.

Philippe Wernet, Professor at the Department of Physics and Astronomy, Uppsala University. Credit: Mikael Wallerstedt
To interpret the complex experimental data, theoreticians from Uppsala University and Stockholm University teamed up and performed advanced quantum-chemical calculations.
“Our calculations allow us to clearly identify how electronic charge flows between the metal catalyst and the C-H group in just the right proportion. We can see how charge flowing from the metal onto the C-H bond glues the two chemical groups together. Charge flowing in the opposite direction instead acts as a scissor that eventually breaks the C and the H atom apart,” explains Ambar Banerjee, Postdoctoral researcher at Uppsala University and lead theoretician of the study.
The study solves a forty-year-old mystery about how an activated catalyst can actually break strong C-H bonds by carefully exchanging fractions of electrons and without the need for huge temperatures or pressures. With their new tool to hand, the researchers next want to learn how to direct the flow of electrons to help develop better catalysts for the chemical industry in order to make something useful out of methane and other alkanes.
Facts
The study builds on the pioneering work of grandfather, father and son Manne, Kai, and Per Siegbahn.
Manne Siegbahn (Uppsala University), who received the Nobel Prize in Physics in 1924, pioneered how different elements can be distinguished by X-rays.
Kai Siegbahn (Uppsala University), who received the Nobel Prize in Physics in 1981, pioneered how different chemical environments of the same element can be distinguished by X-rays.
Per Siegbahn (Stockholm University) theoretically predicted the concerted exchange of electronic charge required for breaking a C-H bond.
Reference: “Tracking C-H activation with orbital resolution” by Raphael M. Jay, Ambar Banerjee, Torsten Leitner, Ru-Pan Wang, Jessica Harich, Robert Stefanuik, Hampus Wikmark, Michael R. Coates, Emma V. Beale, Victoria Kabanova, Abdullah Kahraman, Anna Wach, Dmitry Ozerov, Christopher Arrell, Philip J. M. Johnson, Camelia N. Borca, Claudio Cirelli, Camila Bacellar, Christopher Milne, Nils Huse, Grigory Smolentsev, Thomas Huthwelker, Michael Odelius and Philippe Wernet, 1 June 2023, Science.
DOI: 10.1126/science.adf8042







Totally great this is exactly what I have been imagining and not just in this experiment other catalysts for different bonds, the way for making bonds also.