Scientist at the Max Planck Institute for Experimental Medicine has identified a novel genetic disease mechanism that impacts the strength and timing of neuronal signals, resulting in movement disorders (dyskinesia), attention deficit hyperactivity disorder (ADHD), and autism.
Scientists at the Max Planck Institute for Experimental Medicine in Göttingen reveal that aberrant synapse protein can lead to neurological and psychiatric disorders.
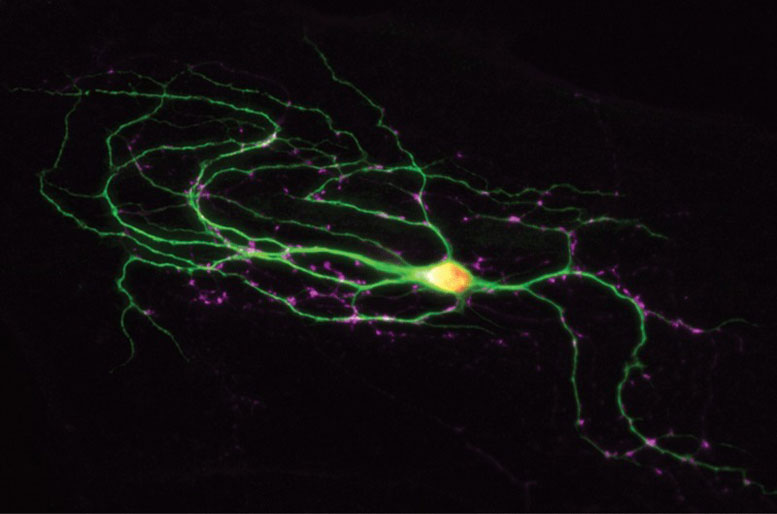
‘Timing is everything’ in the transmission of signals between neurons in the brain. Most of the complex functions that humans are capable of performing would be severely impaired if their neurons were not capable of communicating accurately with one another to a thousandth of a second. Interpersonal communication, learning processes, focusing attention, the rapid processing of sensory stimuli, and even the correct execution of movements would no longer be possible. The Israeli scientist Noa Lipstein-Thoms at the Max Planck Institute for Experimental Medicine in Göttingen has now discovered a new genetic disease mechanism that affects the strength and precise timing of neuronal signals and leads to movement disorders (dyskinesia), attention deficit hyperactivity disorder (ADHD) and autism.
Lipstein-Thoms studies the fundamental mechanisms of signal transmission between neurons. This process takes place at the synapses as they are known. A transmitting neuron triggers the release of a chemical messenger in response to an electrical stimulus. This chemical messenger is recognized by the recipient neuron and converted into an electric signal. A series of regulatory proteins guarantees that this signal transmission process, which is biologically extremely complex, operates with the necessary millisecond precision. The proteins control the release of the messenger at synapses. One of these proteins is known by the cryptic name of Munc13-1.
“Our genetic research on mice has shown that Munc13-1 is vital for the transmission of signals at synapses,” explains Lipstein-Thoms. “When it is missing, the brain does not function because the messenger is blocked and cannot be released at synapses. The affected mouse dies.” Even minor changes to the Munc13-1 protein often has catastrophic consequences because the precise timing of the synaptic signals is lost.
Because of this fundamental importance, Munc13-1 has up until now only been of interest to the basic researchers among the neuroscientists. “Disorders that could only be caused by a malfunction of Munc13-1 were not known,” says Lipstein-Thoms. “My colleagues and I did not expect that the protein could play a role in a disorder as even small disruptions in the functioning of Munc13-1 have grave consequences. We suspected for a long time that a defect in Munc13-1 inevitably led to the death of an organism.”
Munc13-1 as cause of psychiatric disorders
This view has changed dramatically as a result of a new study conducted by Lipstein-Thoms. Together with Göttingen-based neurobiologist Nils Brose and psychiatrists, neurologists, and geneticists at the University of Utrecht in the Netherlands, Lipstein-Thoms describes a patient with a Munc13-1 protein that has been changed by mutation. The patient, who is currently seven years old and is being examined and treated in Utrecht, suffers from an unusual combination of dysfunctional movement patterns, ADHD, and autism.
Lipstein-Thoms’ Dutch colleagues discovered the Munc13-1 mutation during an in-depth genetic examination of the patient. They assume that this mutation is in all probability responsible for the specific symptoms. “This is the first case of a Munc13-1 mutation that is a factor in a disorder. But my colleagues could not explain why this mutation caused the disorder. However, that is the basis on which they can develop a treatment.”
Gene mutations make synapses weaken much faster
“This is where we came in,” says Nils Brose, who supervised Lipstein-Thoms when she was a doctoral student and has worked with her for the last ten years. “Here at the Max Planck Institute for Experimental Medicine we have developed a huge repertoire of methods, reagents, and animal models to analyze very precisely Munc13 proteins and the synapse functions associated with them. And we know a lot about these proteins.” Using the tools and knowledge at her disposal, Lipstein-Thoms demonstrated that the Munc13-1 mutation discovered in the patient initially leads to an unexpected boosting of the synaptic signal transmission but the affected synapses weaken much faster than normal synapses in the case of persistent activity and especially when the activity is intense and very frequent.
“While the changed transmission characteristics of the synapses are rather small, they can explain the complex symptoms in the affected patient,” says Lipstein-Thoms, describing the state of her findings. Many neurological and psychiatric medications already target synapses. “We know which process in the patient’s synapses is damaged and could even try to correct the hyperactivity of synapses that we have described using medications that are already licensed,” explains Lipstein-Thoms. “That would be a wonderful example of how basic research is essential to medical application.”
Reference: “Synaptic UNC13A protein variant causes increased neurotransmission and dyskinetic movement disorder” by Noa Lipstein, Nanda M. Verhoeven-Duif, Francesco E. Michelassi, Nathaniel Calloway, Peter M. van Hasselt, Katarzyna Pienkowska, Gijs van Haaften, Mieke M. van Haelst, Ron van Empelen, Inge Cuppen, Heleen C. van Teeseling, Annemieke M.V. Evelein, Jacob A. Vorstman, Sven Thoms, Olaf Jahn, Karen J. Duran, Glen R. Monroe, Timothy A. Ryan, Holger Taschenberger, Jeremy S. Dittman, Jeong-Seop Rhee, Gepke Visser, Judith J. Jans and Nils Brose, 13 February 2017, Journal of Clinical Investigation.
DOI: 10.1172/JCI90259





Be the first to comment on "Max Planck Scientists Discover a New Genetic Disease Mechanism"