
Lab workers work on infected tissue at the KU Leuven Rega Institute. Credit: Layla Aerts – KU Leuven
Virologists at the Rega Institute at KU Leuven (Belgium) have developed a vaccine candidate against COVID-19 based on the yellow fever vaccine, which as a result also works against yellow fever. Results published today in Nature show that the vaccine protects hamsters from infection with the SARS-CoV-2 coronavirus after a single dose. The vaccine is also effective in monkeys. The team is currently preparing for clinical trials.
To engineer their vaccine, tentatively named RegaVax, the team led by Professor Johan Neyts and Kai Dallmeier inserted the genetic code of the SARS-CoV-2 spikes into the genetic code of the yellow fever vaccine. The researchers tested the vaccine in healthy hamsters and monkeys. Another group of the animals received a placebo.
The researchers first vaccinated the hamsters and then dripped the virus into their noses. Ten days after a single vaccine dose, most of the hamsters were protected against the virus. Three weeks after vaccination, all hamsters were protected. “They also didn’t develop any lung infections. The lungs of the hamsters in the control groups, by contrast, showed clear signs of infection and disease,” Neyts explains.
The team also tested the vaccine in monkeys. “In some of the monkeys, we observed neutralizing antibodies already seven days after vaccination. After fourteen days, high titers of neutralizing antibodies were measured in all animals. This is very fast. Moreover, in the vaccinated animals, the virus was completely or nearly completely gone from their throats.”
Long-lasting immunity
“Ours is the only vaccine currently in development against COVID-19 that also protects against yellow fever,” explains professor Neyts. Previously, the Rega team used the yellow fever vaccine as the foundation for vaccine candidates against Zika, Ebola, and rabies. “The effectiveness and safety of the yellow fever vaccine, which has been in use for 80 years, is well-established. More than 500 million people have already received this vaccine. One dose offers fast protection against yellow fever that in nearly all cases lasts for life.”
“A vaccine that works against COVID-19 and yellow fever could offer an important contribution to the WHO’s campaign to eradicate yellow fever by 2026,” Neyts continues. “Especially now that we know there are mosquito species present in Asia that can transmit the yellow fever virus.”
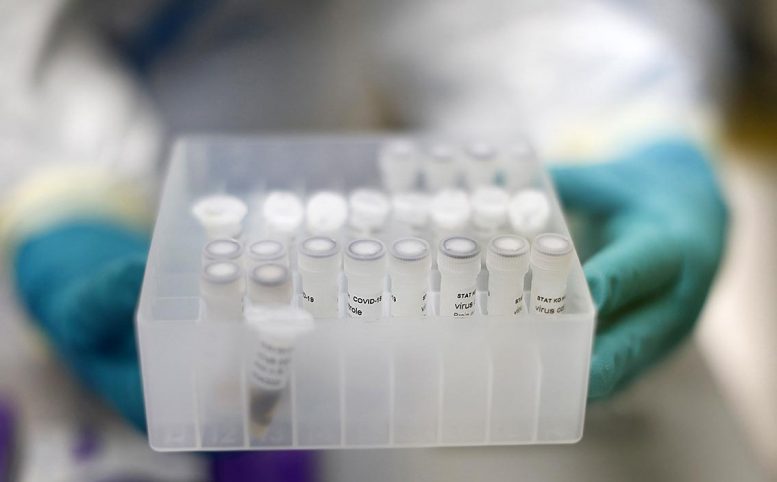
A lab worker holds frozen virus samples at the KU Leuven Rega Institute. Credit: Layla Aerts for KU Leuven
RegaVax works after one dose, unlike many of the front-runners in the race today, which require a repeat vaccination after one month. “This has important logistical implications, in particular for countries with a less advanced medical system,” explains professor Neyts. “Additionally, we expect that the vaccine will offer long-lasting immunity to COVID-19. It could therefore be an ideal candidate for repeat vaccinations when immunity decreases in people who have received one of the first-generation vaccines.”
Finally, the vaccine can be stored at 2-8 °C, while some vaccines require a cold chain with temperatures down to -70 °C. That’s already challenging in the Western world, but it may be nearly impossible to vaccinate large populations in remote tropical and subtropical regions,” Neyts explains.
“An inexpensive, single-dose vaccine that rapidly protects against infection, that can be stored and transported at fridge temperature, and that may, like the yellow fever vaccine on which it is based, result in long-lasting immunity, provides an important and much-needed diversification of the COVID-19 vaccine landscape,” Neyts concludes.
His team is now preparing for clinical trials next year and has joined forces with a specialized and accredited company that will produce the vaccine candidate for testing in humans.
New technique
RegaVax is a vector vaccine: it uses the genetic code of the yellow fever vaccine virus as a carrier (or vector) for the genetic code of the coronavirus spikes. “When working with a related virus, such as the Zika virus, pieces of the genetic code of the yellow fever vaccine virus are swapped with a similar piece of the code of the targeted virus. Using this strategy the team recently developed a Zika vaccine candidate. However, since SARS-CoV-2 is unrelated to yellow fever, a new technology had to be developed to insert an entirely unrelated genetic sequence in the yellow fever vaccine backbone. This concerns an important innovation in the vaccine field.”
Virus inhibitors
“Mind you: vaccines are not a solution for people who are already ill. That is why we are also developing a cure to help COVID-19 patients,” Neyts concludes. “We recently published on the protective activity of the Japanese flu drug favipiravir in hamsters. We have identified some other existing medicines or combinations thereof that inhibit the virus. We are now first exploring their effect in infected hamsters. At the same time, we aim to develop new and powerful virus inhibitors against SARS-CoV-2. For this purpose, we have already tested more than 1.6 million molecules in our fully automated high biosafety laboratory. We’re looking for a needle in a haystack.”
Reference: “A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate” by Lorena Sanchez-Felipe, Thomas Vercruysse, Sapna Sharma, Ji Ma, Viktor Lemmens, Dominique Van Looveren, Mahadesh Prasad Arkalagud Javarappa, Robbert Boudewijns, Bert Malengier-Devlies, Laurens Liesenborghs, Suzanne J. F. Kaptein, Carolien De Keyzer, Lindsey Bervoets, Sarah Debaveye, Madina Rasulova, Laura Seldeslachts, Li-Hsin Li, Sander Jansen, Michael Bright Yakass, Babs E. Verstrepen, Kinga P. Böszörményi, Gwendoline Kiemenyi-Kayere, Nikki van Driel, Osbourne Quaye, Xin Zhang, Sebastiaan ter Horst, Niraj Mishra, Ward Deboutte, Jelle Matthijnssens, Lotte Coelmont, Corinne Vandermeulen, Elisabeth Heylen, Valentijn Vergote, Dominique Schols, Zhongde Wang, Willy Bogers, Thijs Kuiken, Ernst Verschoor, Christopher Cawthorne, Koen Van Laere, Ghislain Opdenakker, Greetje Vande Velde, Birgit Weynand, Dirk E. Teuwen, Patrick Matthys, Johan Neyts, Hendrik Jan Thibaut and Kai Dallmeier, 1 December 2020, Nature.
DOI: 10.1038/s41586-020-3035-9







TheYellow Fever Vaccine is a Weakened Form OF the actual Virus Prevelant in South America…has been used for a long time.
C-19 impact is Severe on on some patients. In Others it is just inconvinient.
Question : Is there a Weakened Version of the Virus already Provided by Mother Nature for those barely impacted by the Virus , whereas a Mutation which impacts some patients attacks the Severe Patintes.
The Cause- Effect Connundrum.
Can we look more Closely at the use of the wekened Version of the Virus as a Potential Virus Vaccine?
The Modified Version of the Yellow Fever may be Comaprable to the Mdified Yellow Fever Vaccine Version.
Since this a already used Vaccine the same may become more acceptable to the Populations across the Globe and can be speeded up for release.
Intuition on Overdrive! Please Check this out Scientists.
so let me guess, big pharma and CDC somehow shut down this clean workable vax