
The research could lead to new strategies for treating Parkinson’s.
A recent study reveals how Parkinson’s spreads throughout the brain.
According to a recent study led by Weill Cornell Medicine scientists, aggregates of the protein alpha-synuclein spread in the brains of Parkinson’s disease patients through a cellular waste-ejection process.
During the process, known as lysosomal exocytosis, neurons release protein waste that cannot be broken down and recycled. The revelation, which was recently published in the journal Nature Communications, might solve one of Parkinson’s disease’s mysteries and lead to new techniques for treating or preventing the neurological disease.
“Our results also suggest that lysosomal exocytosis could be a general mechanism for the disposal of aggregated and degradation-resistant proteins from neurons—in normal, healthy circumstances and in neurodegenerative diseases,” said study senior author Dr. Manu Sharma, an assistant professor of neuroscience in the Feil Family Brain and Mind Research Institute and Appel Alzheimer’s Disease Research Institute at Weill Cornell Medicine.
Parkinson’s is a neurological disorder characterized by the loss of neurons in a certain pattern that spreads across the brain, normally unfolding over decades. The condition is most known for generating hand tremors, muscular rigidity, slower gait, and other movement impairments. However, it affects a wide range of brain regions, resulting in a variety of symptoms, including dementia in the late stages. As of 2022, Parkinson’s disease affects around 1 million individuals in the United States. Because experts still don’t fully understand how the condition progresses, currently available treatments can only alleviate some movement abnormalities but do not stop disease progression.
One important finding that has emerged from the past few decades of Parkinson’s research is that the deaths of neurons in the disease follow the spread, within the brain, of abnormal aggregates of alpha-synuclein, a neuronal protein. This spread is an infection-like, chain-reaction process in which aggregates induce normal alpha-synuclein to join them, and—as they grow larger—break into smaller aggregates that continue to propagate. Experiments in mice and non-human primates have shown that injecting these aggregates into the brain can initiate this spread as well as some Parkinson’s-like neurodegeneration. But the details of how neurons transmit them to other neurons, have never been well understood.
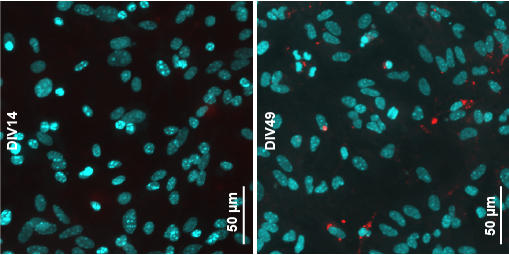
In the study, Dr. Sharma and his team, including co-first author Ying Xue Xie, a doctoral candidate in the Weill Cornell Graduate School of Medical Sciences, showed with detailed studies of Parkinson’s mouse models that alpha-synuclein aggregates—capable of spreading and causing neurodegeneration—originated within neurons. These aggregates, they found, then accumulate within capsule-like waste bins in cells called lysosomes.
Lysosomes contain enzymes that can break down, or “lyse,” proteins and other molecular waste into their building blocks, essentially digesting and recycling them. But the researchers found evidence that alpha-synuclein aggregates, which are knit together with tight bonds in a close-fitting/snugly layered structure called “amyloid”, are not broken down well within lysosomes; instead, they were often found to be simply dumped from their originating neurons. In this process, called exocytosis, the lysosome moves to the cell membrane and merges with it, so that the lysosome contents are discharged—as-is, without any encapsulation—into the fluid surrounding the cell. The finding helps resolve a hotly debated question in the field.
The researchers also showed in further experiments that by reducing the rate of lysosomal exocytosis, they could reduce the apparent concentration of spread-capable aggregates. That, Dr. Sharma said, suggests a future approach to treating Parkinson’s.
“We don’t know yet, but neurons might be better off, even in the long term, if they keep these aggregates inside their lysosomes,” he said. “We see a similar impairment of lysosomal function in some genetic disorders, but these don’t necessarily lead to a Parkinson’s level of disease.”
Dr. Sharma emphasized that prior studies, including genetic studies, have linked lysosomal abnormalities not only to Parkinson’s but to also many other neurodegenerative disorders. This hints that lysosomal exocytosis may be a general mechanism of protein-aggregate spread in these diseases—and potentially a general target for treatments and preventives.
He and his team are currently following up with studies of lysosomes’ roles in Alzheimer’s disease.
Reference: “Lysosomal exocytosis releases pathogenic α-synuclein species from neurons in synucleinopathy models” by Ying Xue Xie, Nima N. Naseri, Jasmine Fels, Parinati Kharel, Yoonmi Na, Diane Lane, Jacqueline Burré and Manu Sharma, 22 August 2022, Nature Communications.
DOI: 10.1038/s41467-022-32625-1
The study was funded by the National Institutes of Health, the Alzheimer’s Association, and the American Federation for Aging Research.




Be the first to comment on "New Discovery Could Resolve a Parkinson’s Disease Mystery"