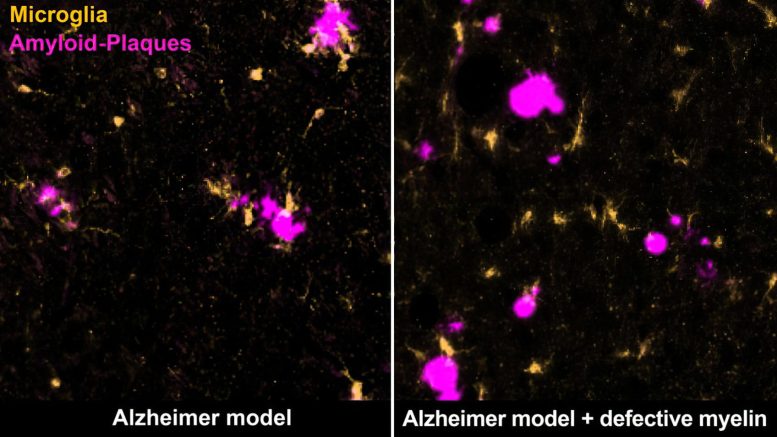
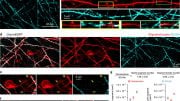
Certain immune cells, microglia (yellow), remove amyloid plaques (magenta) in the brain of the Alzheimer mouse (left). Degenerating myelin distracts them from doing so (right). Credit: Max Planck Institute for Multidisciplinary Sciences
New research has demonstrated that impaired myelin actively promotes disease-related changes in Alzheimer’s disease.
Alzheimer’s disease, an irreversible e type of dementia, is the most prevalent neurodegenerative disease globally. Age is the predominant risk factor for this disease, but the reasons behind this are still not fully understood. However, it is recognized that myelin, the protective sheath around the brain’s nerve cells, undergoes degeneration as one grows older.
A recent study by researchers at the Max Planck Institute (MPI) for Multidisciplinary Sciences in Göttingen has revealed that this compromised myelin directly contributes to the changes observed in Alzheimer’s disease. The findings imply that curbing the degeneration of myelin that comes with age could present new opportunities for preventing the disease or slowing its progression in the future.
What was I about to do? Where did I put the keys? When was that appointment again? It starts with slight memory lapses, followed by increasing problems to orient, following conversations, articulating, or performing simple tasks. In the final phase, patients are most often care-dependent.
Alzheimer’s disease progresses gradually and mainly affects the elderly. The risk of developing Alzheimer’s doubles every five years after the age of 65.
Signs of aging in the brain
“The underlying mechanisms that explain the correlation between age and Alzheimer’s disease have not yet been elucidated,” says Klaus-Armin Nave, director at the MPI for Multidisciplinary Sciences.
With his team of the Department of Neurogenetics, he investigates the function of myelin, the lipid-rich insulating layer of the brain’s nerve cell fibers. Myelin ensures the rapid communication between nerve cells and supports their metabolism.
“Intact myelin is critical for normal brain function. We have shown that age-related changes in myelin promote pathological changes in Alzheimer’s disease,” Nave continues.
In a new study now published in the scientific journal Nature, the scientists explored the possible role of age-related myelin degradation in the development of Alzheimer’s.
Their work focused on a typical feature of the disease: “Alzheimer’s is characterized by the deposition of certain proteins in the brain, the so-called amyloid beta peptides, or Aꞵ peptides for short,” states Constanze Depp, one of the study’s two first authors. “The Aꞵ peptides clump together to form amyloid plaques. In Alzheimer’s patients, these plaques form many years and even decades before the first symptoms appear.” In the course of the disease, nerve cells finally die irreversibly and the transmission of information in the brain is disturbed.
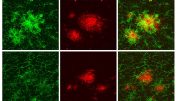
Using imaging and biochemical methods, the scientists examined and compared different mouse models of Alzheimer’s in which amyloid plaques occur in a similar way to those in Alzheimer’s patients. For the first time, however, they studied Alzheimer’s mice that additionally had myelin defects, which also occur in the human brain at an advanced age.
Ting Sun, second first author of the study, describes the results: “We saw that myelin degradation accelerates the deposition of amyloid plaques in the mice’s brains. The defective myelin stresses the nerve fibers, causing them to swell and produce more Aꞵ peptides.”
Overwhelmed immune cells
At the same time, the myelin defects attract the attention of the brain’s immune cells called microglia. “These cells are very vigilant and monitor the brain for any sign of impairment. They can pick up and destroy substances, such as dead cells or cellular components,” Depp adds. Normally, microglia detect and eliminate amyloid plaques, keeping the buildup at bay.
However, when microglia are confronted with both defective myelin and amyloid plaques, they primarily remove the myelin remnants while the plaques continue to accumulate. The researchers suspect that the microglia are ‘distracted’ or overwhelmed by the myelin damage, and thus cannot respond properly to plaques.
The results of the study show, for the first time, that defective myelin in the aging brain increases the risk of Aꞵ peptide deposition. “We hope this will lead to new therapies. If we succeeded in slowing down age-related myelin damage, this could also prevent or slow down Alzheimer’s disease,” Nave says.
Reference: “Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease” by Constanze Depp, Ting Sun, Andrew Octavian Sasmita, Lena Spieth, Stefan A. Berghoff, Taisiia Nazarenko, Katharina Overhoff, Agnes A. Steixner-Kumar, Swati Subramanian, Sahab Arinrad, Torben Ruhwedel, Wiebke Möbius, Sandra Göbbels, Gesine Saher, Hauke B. Werner, Alkmini Damkou, Silvia Zampar, Oliver Wirths, Maik Thalmann, Mikael Simons, Takashi Saito, Takaomi Saido, Dilja Krueger-Burg, Riki Kawaguchi, Michael Willem, Christian Haass, Daniel Geschwind, Hannelore Ehrenreich, Ruth Stassart and Klaus-Armin Nave, 31 May 2023, Nature.
DOI: 10.1038/s41586-023-06120-6


With a family history of dementia and a personal history of two disparate incidents of nutritional deficiency related temporary short-term memory problems, I don’t recall exactly when I (as only a lay victim, investigator, experimenter and discoverer) first postulated that high blood serum levels of uric acid erode cholesterol from the myelin sheaths of brain and nerve cells but it was probably at least two decades ago.
I now perceive intimate relationships between Dr. Arthur F. Coca’s (by 1935; my) kind of long-term chronic nearly subclinical non-IgE-mediated food allergy reactions, added ‘cultured-free’ (can cross the blood/brain barrier) monosodium glutamate (MSG; FDA approved for expanded use as an alleged ‘flavor enhancer’ in the US in 1980), urine pH (slightly alkaline being the published ‘healthy-normal’) and too-common medical errors (e.g., undiagnosed calcium deficiency, because the body reallocates calcium to try to maintain the blood at an optimal pH and standard blood serum testing for calcium is unreliable).
Trusting the researchers at MPI have hit the nail on the head with their “…defective myelin in the aging brain increases the risk of AB peptide deposition.” I additionally submit the role of added MSG is to over excite the brain/immune response to cause practically harmless individual food allergy reactions to become chronic and deadly dangerous long-term (highly individual; many individual variables).
As to better treatments and prevention, I submit to permanently ban the use of added MSG could bring an immediate decline in the number of cases and severity of dementia, as well as to devise and implement a new method, and/or resurrect the “cytotoxic blood testing for food allergies” I had in late 1981, could inform multitudes of people of very, very mild food allergy reactions they are presently experiencing which could be avoided.
Bottom line: fatally flawed “evidence-based” medicine/science is no substitute for even minimally qualified “experience-based” of the same. And, professional bias against lay discovery is no good reason to instigate and maintain multiple epidemics of cheaply and easily prevented chronic diseases.