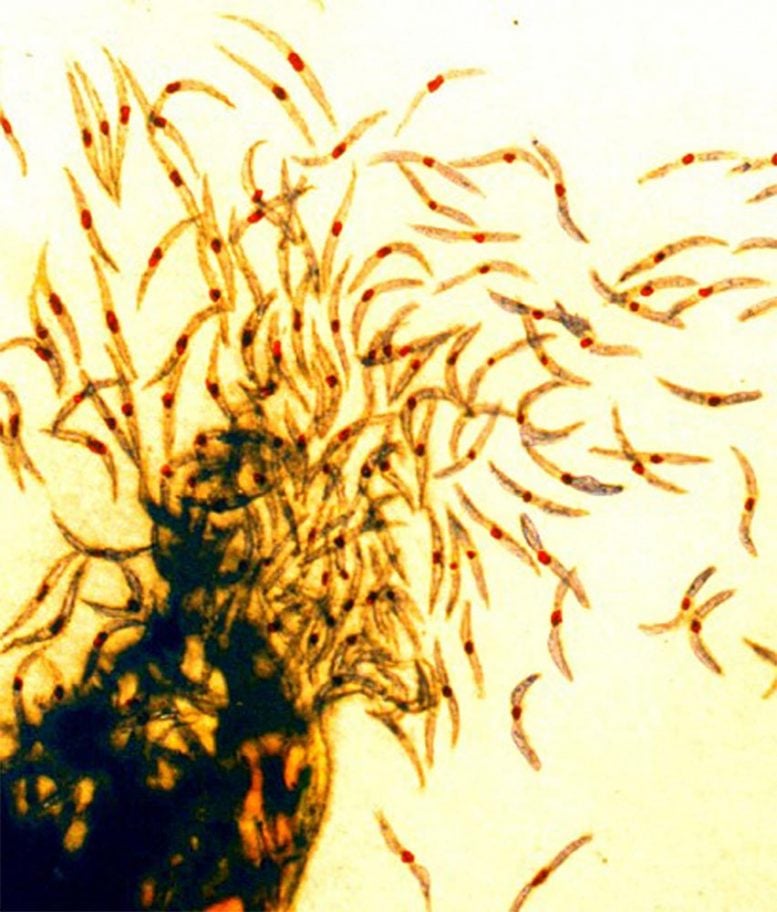
Malaria sporozoites, the infectious form of the malaria parasite that is injected into people by mosquitoes. Credit: NIAID
Findings published in Nature offer potential for use by travelers and prevention of malaria in African populations in near future.
Researchers from Sanaria® Inc. and the National Institutes of Health (NIH) are making progress in the development of highly protective malaria vaccines.
In an article published today (June 30, 2021) in Nature, Sanaria’s PfSPZ-CVac (CQ) vaccine is reported as being safe and protecting 100% of six subjects against a variant malaria parasite three months after their last dose in the company’s Phase 1 safety and efficacy trial. This is the first time complete protection against a variant malaria parasite has ever been achieved that long after vaccine administration.
The variant parasite used in the trial is a Brazilian malaria parasite genetically more variant from the African parasites in the vaccine than 700 malaria parasites from Africa. Protection was achieved at a dose that is 20% of the company’s first-generation malaria vaccine dosage.
“These results represent extremely important progress, unanticipated by most malaria experts,” said Professor Martin Grobusch, Head of the Center of Tropical Medicine and Travel Medicine, Amsterdam University Medical Centers. “Until recently, malaria vaccine developers sought to achieve high-level protection against non-variant malaria parasites, often only two to three weeks after vaccination, with immunity waning thereafter. The finding of 100% protection against variant parasites that are so divergent from the vaccine parasites at three months is unprecedented. This vaccine approach should be advanced now as a potential tool to protect travelers to Africa and further developed for the prevention of malaria in African populations.”
The Nature paper also includes the results of a second study using PfSPZ-CVac (PYR), which combines Sanaria’s PfSPZ with pyrimethine (PYR), a drug used for seasonal malaria prevention in African preschoolers. This vaccine was well tolerated and protected 82% of the 17 subjects to whom it was administered from the Brazilian variant parasites or the African vaccine parasites three months after their last dose.
“We are encouraged by the significant findings reported in this seminal paper, which justify our investment in Sanaria and its systematic, scientifically sound approach to developing the highly protective, cost-effective vaccines required to eliminate malaria, a scourge of humanity, particularly for the most underserved on our planet,” said Holm Keller, Co-Managing Director, the EU Malaria Fund.
“Sanaria’s vaccine development program is designed to produce safe, cost-effective vaccines that provide high-level protection against malaria parasites that cause more than 400,000 deaths annually, primarily in Africa,” said Stephen L. Hoffman, Sanaria’s CEO. “With this goal in mind, Sanaria and our partners in the International PfSPZ Consortium have pursued a step-by-step approach to maintaining safety, increasing efficacy toward 100% against variant parasites, increasing the durability of efficacy, and decreasing the required vaccine dosage. This study reports huge progress in all four areas.”
Sanaria® PfSPZ-CVac is a chemo-attenuated, live whole parasite vaccine in which an anti-malarial drug is co-administered with parasite cells (PfSPZ) to kill them before a clinical infection develops. In the trial reported in Nature, the anti-malarial was either chloroquine (CQ) or PYR and efficacy was measured by controlled human malaria infection (CHMI). In addition to exposure in natural settings in Africa, the company has relied on CHMI of vaccinated and unvaccinated adults to assess vaccine efficacy. This is a rigorous test of malaria vaccines that can be conducted with small numbers of trial participants since 100% of unvaccinated subjects develop malaria.
Reference: “Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity” by Agnes Mwakingwe-Omari, Sara A. Healy, Jacquelyn Lane, David M. Cook, Sahand Kalhori, Charles Wyatt, Aarti Kolluri, Omely Marte-Salcedo, Alemush Imeru, Martha Nason, Lei K. Ding, Hope Decederfelt, Junhui Duan, Jillian Neal, Jacob Raiten, Grace Lee, Jen C. C. Hume, Jihyun E. Jeon, Ijeoma Ikpeama, Natasha KC, Sumana Chakravarty, Tooba Murshedkar, L. W. Preston Church, Anita Manoj, Anusha Gunasekera, Charles Anderson, Sean C. Murphy, Sandra March, Sangeeta N. Bhatia, Eric R. James, Peter F. Billingsley, B. Kim Lee Sim, Thomas L. Richie, Irfan Zaidi, Stephen L. Hoffman and Patrick E. Duffy, 30 June 2021, Nature.
DOI: 10.1038/s41586-021-03684-z
The clinical trial was sponsored by Sanaria Inc. and conducted by the LMIV, NIAID and NIH.
About Sanaria Inc.: Sanaria is a biotechnology company based in Rockville, Maryland (USA) whose mission is development of whole parasite PfSPZ vaccines to protect against malaria. Sanaria’s vaccines have been highly protective against malaria in clinical trials in the US, Africa, and Europe. Sanaria’s vaccines will be used once cleared by regulatory agencies to prevent malaria in individuals and in combination with other malaria control measures to stop malaria transmission and eliminate malaria in defined geographic regions.
About NIH, NIAID, LMIV: The LMIV was created in 2009 to conduct basic and applied research relevant to malaria immunology and vaccine development, to pursue novel vaccine concepts, or produce prototype malaria vaccines, and to conduct early-phase clinical trials of promising vaccine candidates. Its overarching goal is to develop malaria vaccines that will reduce severe disease and death among African children and pregnant women and will eliminate malaria from low-transmission areas of the world.







Be the first to comment on "New Malaria Vaccine Shows Unprecedented Protection – Safe, Strong, and Lasting"