When malformed red blood cells clump together, obstructing small blood capillaries and causing extreme discomfort and swelling in the afflicted body areas, this is one of the most common complications of sickle-cell disease. A recent MIT study gives light on the causes of these occurrences. In addition, the findings offer a step toward predicting when such a catastrophe would arise.
One of the most common complications of sickle-cell disease occurs when deformed red blood cells clump together, blocking tiny blood vessels and causing severe pain and swelling in the affected body parts.
A new study from MIT sheds light on how these events, known as vaso-occlusive pain crises, arise. The findings also represent a step toward being able to predict when such a crisis might occur.
“These painful crises are very much unpredictable. In a sense, we understand why they happen, but we don’t have a good way to predict them yet,” says Ming Dao, a principal research scientist in MIT’s Department of Materials Science and Engineering and one of the senior authors of the study.
The researchers found that these painful events are most likely to be produced by immature red blood cells, called reticulocytes, which are more prone to stick to blood vessel walls.
Subra Suresh, president of Singapore’s Nanyang Technological University, former dean of engineering at MIT, and the Vannevar Bush Professor Emeritus of Engineering, is also a senior author of the study, which appears in Proceedings of the National Academy of Sciences the week of September 3. The paper’s lead authors are MIT postdoc Dimitrios Papageorgiou and former postdoc Sabia Abidi.

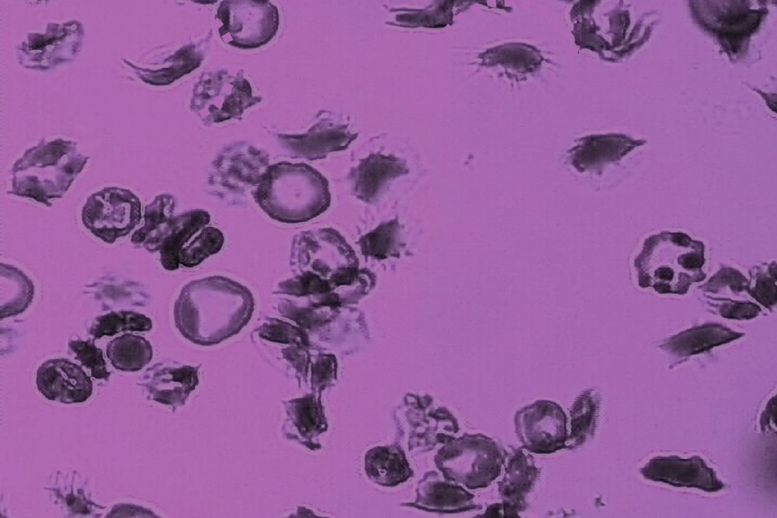
V1: Different types of adherent sickle cells to the microchannel surface under hypoxia (low oxygen) and shear flow, including i) sickle reticulocytes (young red blood cells): a, b; ii) sickle mature red blood cells: d, g, h, i, f; and iii) irreversibly sickled cells: m. (Credit: Courtesy of the researchers)
Simulating blood flow
Patients with sickle cell disease have a single mutation in the gene that encodes hemoglobin, the protein that allows red blood cells to carry oxygen. This produces misshapen red blood cells: Instead of the characteristic disc shape, cells become sickle-shaped, especially in low-oxygen conditions. Patients often suffer from anemia because the abnormal hemoglobin can’t carry as much oxygen, as well as from vaso-occlusive pain crises, which are usually treated with opioids or other drugs.
To probe how red blood cells interact with blood vessels to set off a vaso-occlusive crisis, the researchers built a specialized microfluidic system that mimics the post-capillary vessels, which carry deoxygenated blood away from the capillaries. These vessels, about 10-20 microns in diameter, are where vaso-occlusions are most likely to occur.

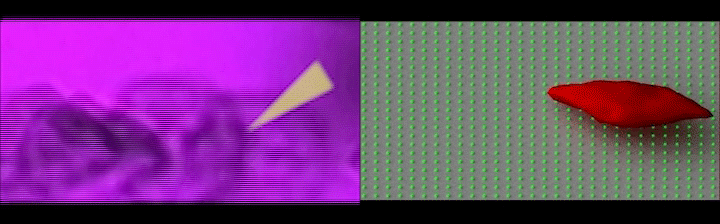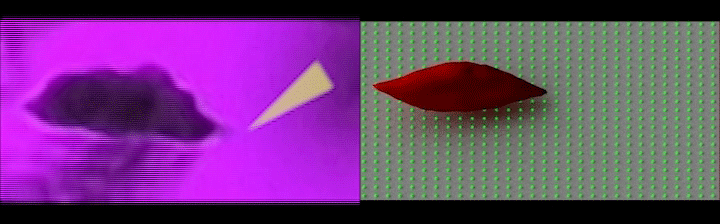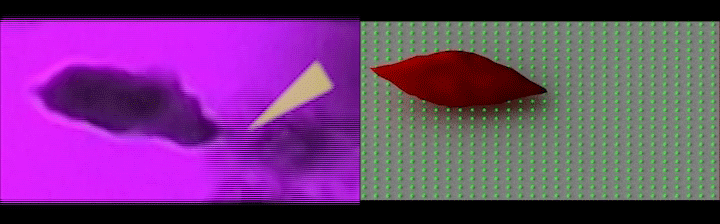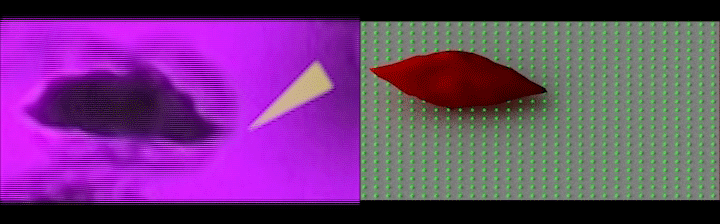
V2: Left: Simultaneous adhesion & polymerization under low oxygen of a sickle reticulocyte (young red blood cell), showing multiple sickle hemoglobin fibers growing out of cell bulk; Right: The same adherent sickle reticulocyte after hypoxia-to-reoxygenation cycle, showing polymerized hemoglobin fiber dissolution/retraction and residual adhesion sites. (Credit: Courtesy of the researchers)
The microfluidic system is designed to allow the researchers to control the oxygen level. They found that when oxygen is very low, or under hypoxia, similar to what is seen in post-capillary vessels, sickle red cells are two to four times more likely to get stuck to the blood vessel walls than they are at normal oxygen levels.
When oxygen is low, hemoglobin inside the sickle cells forms stiff fibers that grow and push the cell membrane outward. These fibers also help the cells stick more firmly to the lining of the blood vessel.
“There has been little understanding of why, under hypoxia, there is much more adhesion,” Suresh says. “The experiments of this study provide some key insights into the processes and mechanisms responsible for increased adhesion.”
The researchers also found that in patients with sickle cell disease, immature red blood cells called reticulocytes are most likely to adhere to blood vessels. These young sickle red cells, just released from bone marrow, carry more cell membrane surface area than mature red blood cells, allowing them to create more adhesion sites.
“We observed the growth of sickle hemoglobin fibers stretching reticulocytes within minutes,” Papageorgiou says. “It looks like they’re trying to grab more of the surface and adhere more strongly.”
Left: Simultaneous adhesion & polymerization of an irreversibly sickled cell under low oxygen, where the cell adheres to the surface and flips around the adhesion site aligning with the flow direction; Right: Computer simulation of the adhesion of an irreversibly sickled cell under shear flow, where the green dots represent an array of adhesion sites on the surface. (Credit: Courtesy of the researchers)
Patient predictions
The researchers now hope to devise a more complete model of vaso-occlusion that combines their new findings on adhesion with previous work in which they measured how long it takes blood cells from sickle cell patients to stiffen, making them more likely to block blood flow in tiny blood vessels. Not all patients with sickle cell disease experience vaso-occlusion, and the frequency of attacks can vary widely between patients. The MIT researchers hope that their findings may help them to devise a way to predict these crises for individual patients.
“Blood cell adhesion is indeed a very complex process, and we had to develop new models based on such microfluidic experiments. These adhesion experiments and corresponding simulations for sickle red cells under hypoxia are quantitative and unique,” says George Karniadakis, a professor of applied mathematics at Brown University and a senior author of the study.
“The work done on sickle cell disease by Dao and Suresh over the last decade is remarkable,” says Antoine Jerusalem, an associate professor of engineering science at the University of Oxford who was not involved in the research. “This paper in particular couples numerical and experimental state-of-the-art techniques to enhance the understanding of polymerization and adhesion of these cells under hypoxia, a drastic step towards the elucidation of how vaso-occlusion can arise in sickle cell disease.”
The research was funded by the National Institutes of Health.
Reference: “Simultaneous polymerization and adhesion under hypoxia in sickle cell disease” by Dimitrios P. Papageorgiou, Sabia Z. Abidi, Hung-Yu Chang, Xuejin Li, Gregory J. Kato, George E. Karniadakis, Subra Suresh and Ming Dao, 6 September 2018, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.1807405115



Be the first to comment on "New Research Shows How Sickled Red Blood Cells Clump Together"