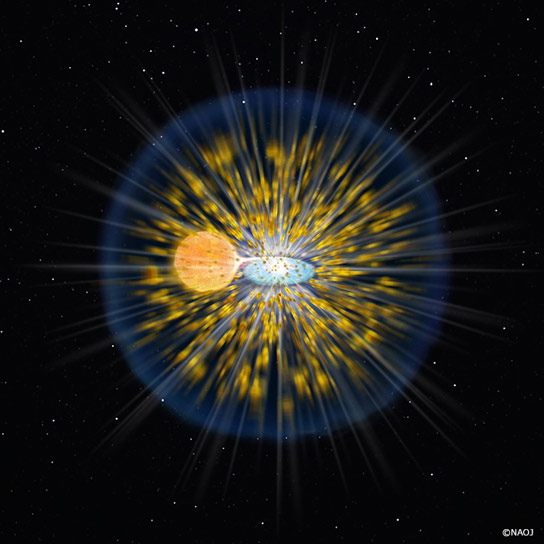
Figure 1: Artist’s rendition of a classical nova explosion (Credit: NAOJ) A classical nova explosion is thought to occur on the surface of a white dwarf (center right) with a close companion star (center left; a sun-like main sequence or more evolved star). When the distance between two stars is close enough, the outer gas of the companion starts to accumulate on the surface of the white dwarf via an accretion disk. The thicker gas layer on the white dwarf increases its temperature and density. Then, nuclear reactions occur in a different way from those inside stars. In the case of stellar interiors, the huge energy produced by nuclear reactions in the core is balanced by the gravity of the surrounding gas, and then the reaction becomes stable. However, the nuclear reaction in a thin gas layer on the surface of a white dwarf has a different result. It becomes a runaway nuclear reaction, and results in an explosion that blows away the gas layer. Credit: National Astronomical Observatory of Japan
Using the 8.2-meter Subaru Telescope High Dispersion Spectrograph (HDS), a team of astronomers observed Nova Delphini 2013 and discovered that the outburst is producing a large amount of lithium.
A team of astronomers from National Astronomical Observatory of Japan (NAOJ), Osaka Kyoiku University, Nagoya University, and Kyoto Sangyo University observed Nova Delphini 2013 (Figure 1, 3) which occurred on August 14, 2013. Using the 8.2-meter Subaru Telescope High Dispersion Spectrograph (HDS) to observe this object, they discovered that the outburst is producing a large amount of lithium (Li; Note 1). Lithium is a key element in the study of the chemical evolution of the universe because it likely was and is produced in several ways: through Big Bang nucleosynthesis, in collisions between energetic cosmic rays and the interstellar medium, inside stellar interiors, and as a result of novae and supernova explosions. This new observation provides the first direct evidence for the supply of Li from stellar objects to the galactic medium. The team hopes to deepen the understanding of galactic chemical evolution, given that nova explosions must be important suppliers of Li in the current universe.
Lithium: the Key to Understanding the Nucleosynthesis in the Universe
The universe consisted primarily of hydrogen (H) and helium (He) immediately after the Big Bang except for very small amounts of Li. Since there are other elements heavier than H and He in the universe now, astronomers want to understand how the heavy elements – such as carbon (C), oxygen (O), and iron (Fe) (which are present in our bodies) – are produced. Such heavy elements are mainly produced in stellar interiors or supernovae. Then, they are supplied to the interstellar medium as seed materials for the next generation of stars.
Li is the third lightest element following H and He, and is familiar to us as the base material for the Li-ion batteries used in PCs, smart phones, eco-cars, etc. Big Bang nucleosynthesis produced a very small amount of Li (Note 2). Collisions between galactic cosmic rays (energetic atomic nuclei traveling with very high speeds) and atomic nuclei in the interstellar medium are also assumed to produce Li by breaking heavy elements’ nuclei (e.g., C, O). Low-mass stars like the Sun, and events such as supernova explosions are also considered as candidates of Li production sites. Furthermore, scientists have been assuming that novae should also produce this element (Figure 2).
Because many sites and events can produce Li as described above, Li is the best indicator to probe the complete chemical evolution of the universe. Many scientists have studied this element by measuring the amount of Li found in various stars in our galaxy. This allowed them to estimate the amount produced through each process. Today, as a result of these indirect approaches, low-mass stars or nova explosions are thought to be the most important candidates for Li production in the current galaxy epoch. (Note 2). However, there have been no direct observations of the processes (Note 3).
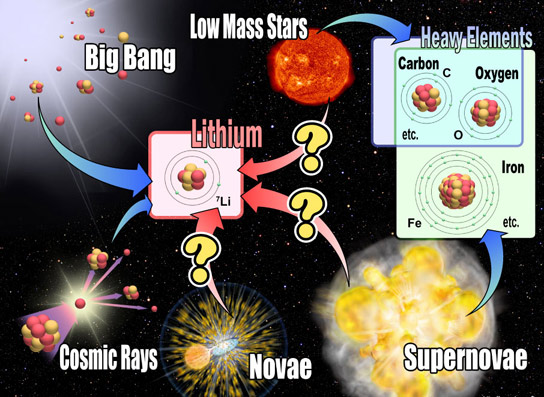
Figure 2: Nucleosynthesis in the universe (Credit: NAOJ) Heavy elements such as C, O, and Fe are mainly produced in stellar interior and/or supernova. On the other hand, Li might be produced in many other ways: in the Big Bang, galactic cosmic ray collisions. Li production in stellar originating objects has not been confirmed yet by observations, as designated by “?” marks. Credit: National Astronomical Observatory of Japan
Nova Delphini 2013
On August 14th, 2013, the well-known Japanese amateur astronomer Koichi Itagaki found a bright new star in the constellation Delphinus (Figure 3). This star, which was named Nova Delphini 2013 (=V339 Del), was at magnitude 6.8 at discovery and peaked at 4.3 mag within two days. It was the first naked-eye nova since 2007, when V1280 Sco was found. About 40 days later, in September 2013, a team of astronomers observed the nova to investigate the materials expelled by the explosion. That is when they found that the nova produced a large amount of Li.
Nova Delphini 2013 is considered one of the “classical novae.” These brighten when explosive nuclear reactions occur in materials accumulated on the surface of a white dwarf star in a close binary system. The nuclear reactions are thought to produce a different series of elements (compared to those produced in stellar interiors or supernova explosions). Li is assumed to be an element typically produced in such outbursts. Historically, no one has been able to get good observational evidence for its production in nova explosions.

Figure 3: Discovery images of Nova Delphini 2013 (Credit: Koichi Itagaki) The upper left panel is before the explosion (about 1 day). The upper right shows the nova after the explosion. The bottom is the confirmation image taken with the 60-cm telescope. This nova is an object within our galaxy. Its distance is about 14,000 light-years. The nova became about 150,000 times brighter at the maximum, compared with old pre-explosion images. Credit: National Astronomical Observatory of Japan
Discovery of a Beryllium Isotope (7Be) to Form Lithium in Nova Spectra
When the research group observed Nova Delphini 2013 using the Subaru Telescope, they used the High Dispersion Spectrograph to discern the constituents of the expelled materials from the nova explosion at four epochs (Figure 4).
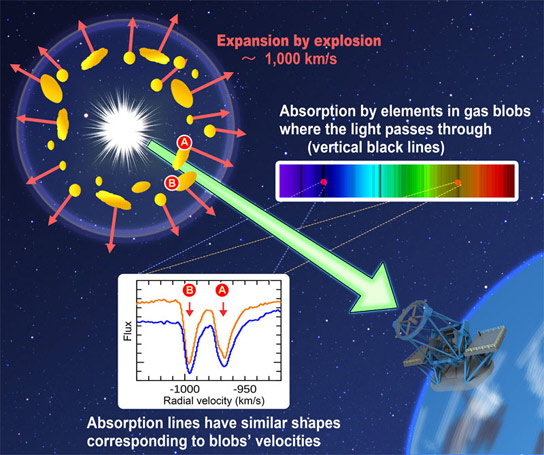
Figure 4: How does the light from the nova reach observers? (Credit: NAOJ) After the explosion, the material in the region around the white dwarf is very hot and has a very high density. The light radiated by the white dwarf passes through some of the gaseous blobs blown away by the explosion, and that light reaches observers. Each element in the blobs absorbs the light at a specific wavelength. Because each blob has a different velocity (~1000 km/s), the nova spectrum shows many groups of weak absorption lines created by various elements. Credit: National Astronomical Observatory of Japan
Absorption lines originating from many elements such as H, He, and Fe are identified in the observed spectra (Note 4). Among them, there are sets of strong absorption lines in the ultraviolet (UV) range (wavelength ~313 nanometers) of the spectrum (Note 5). Comparing these lines with other lines originating from H, calcium (Ca), and other elements, it turns out that they are originating from an isotope of beryllium (Be), 7Be, which is the fourth-lightest element in the universe (Figure 5).
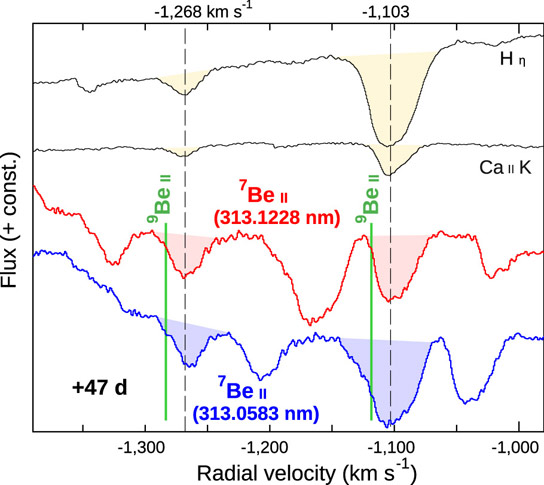
Figure 5: The absorption lines originating from hydrogen (Hη), singly ionized calcium (Ca II K), and the doublet originating from singly ionized 7Be (red and blue) in the observed spectrum (Credit: NAOJ) The spectrum was taken with HDS 47 days after the explosion. The vertical axis shows the flux (+ constants for offsets). The horizontal axis shows the radial velocity (kilometers per second) calculated from the rest wavelengths of each absorption line. It is found that all lines have two components of velocities at -1268 and -1103 km/s. Furthermore, the Be absorption lines clearly show that they originate from a radioactive isotope 7Be, instead of the only stable isotope 9Be (green vertical lines; Note 6). Credit: National Astronomical Observatory of Japan
In a classical nova, the isotopes of He (3He) and plentiful 4He transferring from the companion are fused together to form radioactive 7Be in a very high-temperature environment on the surface of a white dwarf. This radioactive isotope decays to form an isotope of lithium (7Li) within a short time (half-life of 53.22 days) (Figure 6). Because 7Li is very fragile in a high-temperature environment, it is necessary to transport 7Be to a cooler region in order to enrich Li in the interstellar medium. Novae completely fill this requirement. Therefore, they are assumed to be strong candidates as suppliers of Li in the universe.
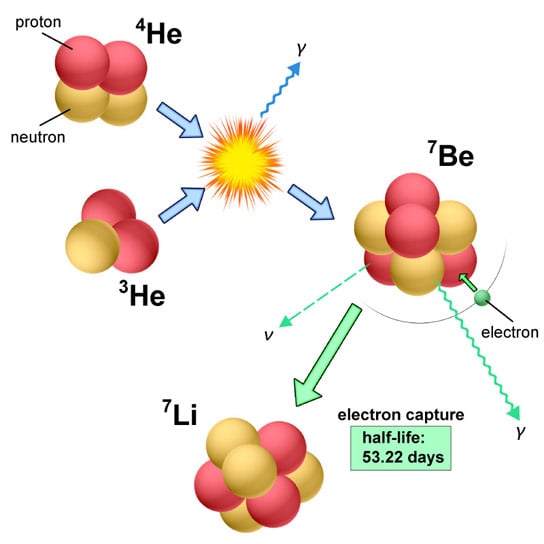
Figure 6: Nuclear reactions to form 7Be, and then 7Li in classical nova explosions (Credit: NAOJ) At the time of an explosion, 3He and 4He are fused to form 7Be (blue arrows). Then, 7Be gradually decays into 7Li (via electron capture) in gas blobs blown away by explosive winds (green arrows). Credit: National Astronomical Observatory of Japan
This discovery of 7Be within 50 days after the Nova explosion means that this explosion is actually producing a large amount of 7Li formed from 7Be. Because 7Be is found in the gas blobs blown away from the central region of the nova at high velocities (~1000 km/s or ~620 mi/s), 7Li formed from this 7Be should not be destroyed in a high-temperature environment. This 7Li spreads into interstellar space, and will be included in the next generation of stars. It is found that the 7Be abundance in the gas blobs estimated from the strengths of their absorption lines is comparable to that of Ca. This amount of 7Be (= 7Li) should be quite large, given that Li is known as a very rare element in the universe (Note 7).
Impact of this Research
The amount of Li rapidly increases in the galaxy in the current epoch, where the amounts of heavy elements have increased. Therefore, it has long been speculated that low-mass stars with longer lifetimes should be among the major suppliers of Li in the universe. Because nova explosions occur in binary systems evolved from such low-mass stars (especially 3He-rich companion, which is necessary to produce 7Be), they are strong candidates as Li suppliers. The observations made using the Subaru HDS provide the first strong evidence to prove that novae produce significant amounts of Li in the universe. This discovery confirms the chemical evolution model from the Big Bang to the present universe, as predicted by scientists.
Furthermore, the observed amount of Li produced in this nova explosion is proven to be higher than predicted by theoretical estimates. Nova Delphini 2013 shows rather typical characteristics of classical novae. If other novae also produce a large amount of Li as Nova Delphini 2013 did, nova explosions must be recognized as very major Li factories in the universe. In the near future, more observations of other nova explosions will provide a much clearer model of Li evolution.
Notes:
- Lithium is composed of two stable isotopes – 6Li and 7Li. In the solar system, about 92 percent of Li is 7Li. In this press release, “Li” means the most abundant 7Li.
- Many scientists have tried to measure Li abundances in various stars in the galaxy to investigate the origin of Li in the universe. Figure 7 displays a schematic diagram of their results. Stars with amounts of low heavy elements were born in the early universe. Big Bang is presumably the primary source of Li in these stars. Indeed, their Li abundance is almost constant independent of the amount of other elements like iron. However, the value is known to be a few times lower than the theoretical prediction for the Big Bang nucleosynthesis. Many scientists have been working to solve this problem. On the other hand, stars with higher abundance of heavy elements were born in the more recent universe (> a few Gyrs from the Big Bang). They look to have significantly more Li. To explain this rapid increase in Li, astronomers have assumed that Li production in low-mass stars or nova explosions should be dominant in the universe today, surpassing production in supernovae or in the interstellar medium.
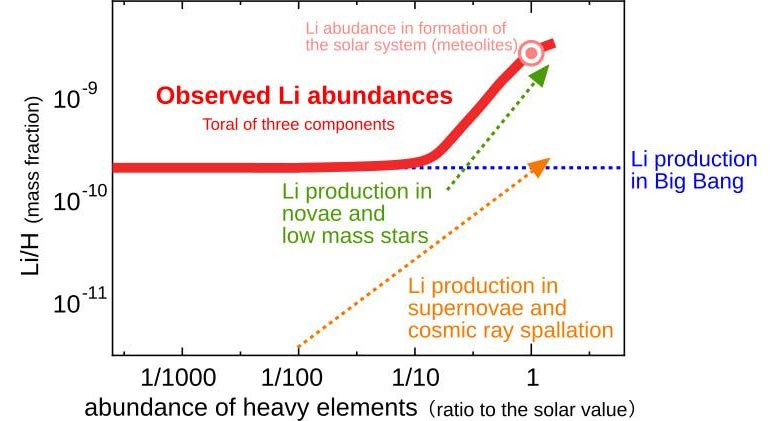
Figure 7: Schematic diagram of Li evolution in the universe The vertical axis shows the number ratio of Li and H. The horizontal axis shows the amount of heavy elements (ratio to solar abundance). The farther to the right a star lies in the diagram, the more heavy elements it has and the younger it is. The red curve in the diagram presents the upper envelope of the observed Li abundance [Reference: Prantzos, N., A&A 542, A67 (2012)]. To explain the shape of this curve, scientists assume that there are three sources for Li production: (1) nucleosynthesis in the Big Bang (should be a constant in the diagram = blue), (2) Li production in objects or events originating from massive stars (e.g. supernova explosions, spallation of nuclei triggered by galactic cosmic rays), which begins its contribution in the early universe (orange), and (3) Li production in low-mass (=longer lifetime) stars (e.g. nova explosions), which begins in the recent universe, where the amount of heavy elements exceeds 10 percent of the solar value (green). In particular, the contribution of the third component must be dominant in the current universe, though scientists have been unable to get any signs of Li production in low-mass stellar components. Credit: NAOJ
- Some low-mass (a few solar masses) evolved stars have been found to have Li-enriched surfaces. They are also possible Li-suppliers in the universe. However, Li should be destroyed within a high temperature environment (hotter than 2,500,000 K or 4,500,000 F). If the Li production in these stars is stopped at once, such Li could be easily depleted by interior convection. Therefore, it is still unknown how these stars contribute to Li-enrichment in the interstellar medium.
- The most brilliant features in the HDS spectrum obtained on 38 days after the explosion are many broad emission lines originating from H, He, Fe, and other species in the diffuse expanding gas (upper diagram in Figure 8). Zooming into each emission line, the research group found that each line has a similar set of weak absorption lines on its blueward wing.
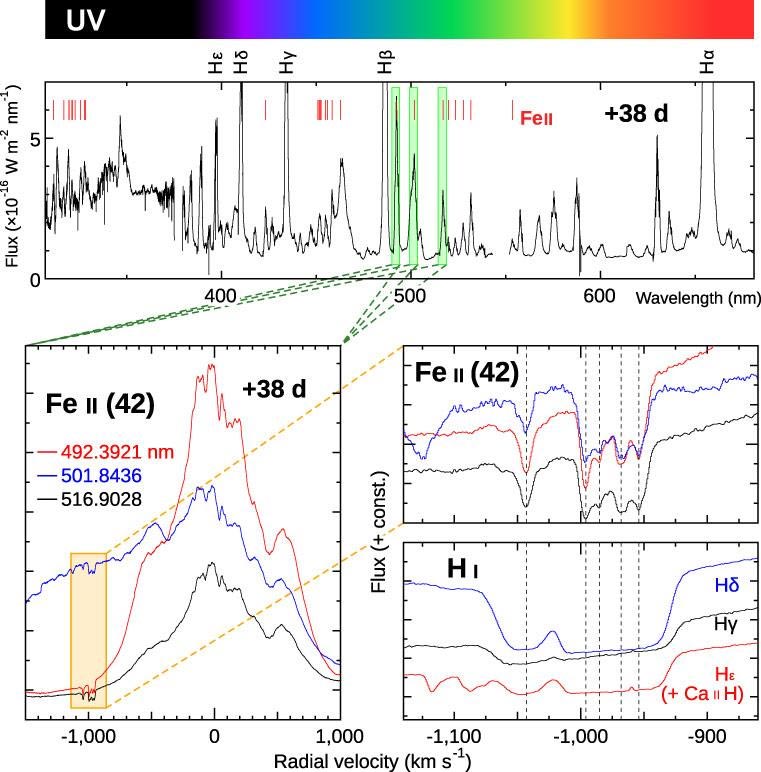
Figure 8: HDS spectrum taken on 38 days after the explosion Each vertical axis shows the flux of the spectrum. The horizontal axis in the top panel shows the wavelength (in nanometers). Those in the three lower panels show the radial velocities (in kilometers per second) calculated from the rest wavelengths of each line. Zooming into several emission lines originating from singly ionized iron (Fe II), there is a set of weak absorption lines with common velocity components. Such weak absorption lines are also found in other lines originating from H or other species. Credit: NAOJ
- Figure 8 (to the left of the upper diagram) shows that HDS has enough sensitivity even in the UV range (wavelength < 400 nanometers). This is achieved by the combination of the excellent site location (high altitude = 4200 meters or 13,800 feet), the large aperture of the Subaru Telescope, and the high UV sensitivity of the detectors. There are only a few instruments in the world that achieve good quality spectra in the UV range. In Nova Delphini 2013, enormous sets of weak absorption lines mentioned in Figure 8 and Figure 9 are found in this wavelength range.
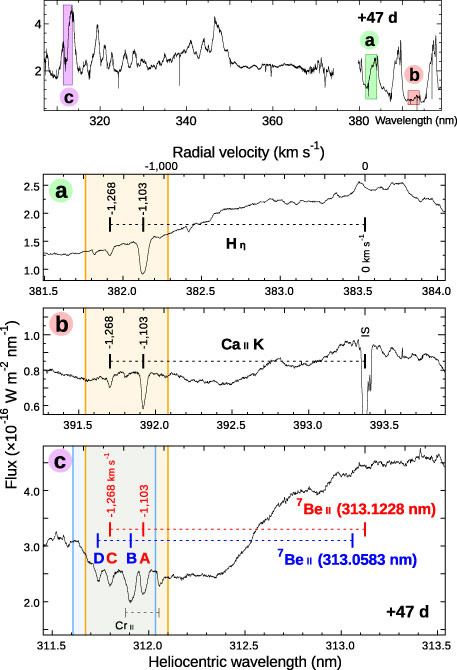
Figure 9: HDS spectrum in UV range (47 days after the explosion) (Credit : NAOJ) The lower three panels (a, b, and c) show the enlarged views of the colored region in the top panel. Each vertical axis shows the flux of the spectrum. Each horizontal axis shows its wavelength (in nanometers). The three lower panels are shown in the same radial velocity scale (the upper horizontal axis in kilometers per second). It is clear that there are same blue-shifted components in hydrogen (Hη), calcium (Ca II K), and beryllium (Be II). Credit: NAOJ
- Be has only one stable isotope, 9Be, in the universe. However, the absorption lines in 313 nanometers are found to be originating from the other isotope, 7Be, instead of the stable 9Be. 7Be is a radioactive isotope, which decays to form 7Li within a short time (half-life: 53 days). Since the 1970s, scientists have theorized that this isotope is produced in nova explosions or at other sites in the galaxy [reference: e.g., Cameron, A. G. W. & Fowler, W. A., ApJ 164, 111-114 (1971)]. However, nobody could find this isotope in such candidate sites because of its very short lifetime.
- The abundance of 7Be estimated from the strengths of absorption lines is found to be about 0.04 percent of the total expelled mass in this nova explosion (~0.000000006 percent in the solar surface, ~0.002 percent in the earth’s crust). This abundance is about six times larger than those from theoretical estimates.
Reference: “Explosive lithium production in the classical nova V339 Del (Nova Delphini 2013)” by Akito Tajitsu, Kozo Sadakane, Hiroyuki Naito, Akir Arai and Wako Aoki, 18 February 2015, Nature.
DOI:10.1038/nature14161
arXiv: 1502.05598





Companion star feeding a gas shroud around a white dwarf producing lithium charged ionization responsible for Halls whistler lightning effect making an explosion on the surrounding producing lightning explosion
Hence atypical fusion technology could be developed by shrouding lithium ions to simulate a gas thrust producing whistler wave explosions to ignite the plasma
they discovered that the outburst is producing a large amount of lithium (Li; Note 1). Lithium is a key element in the study of the chemical evolution of the universe because it likely was and is produced in several ways: through Big Bang nucleosynthesis, in collisions between energetic cosmic rays and the interstellar medium, inside stellar interiors, and as a result of novae and supernova explosions. This new observation provides the first direct evidence for the supply of Li from stellar objects to the galactic medium.
A classical nova explosion is thought to occur on the surface of a white dwarf (center right) with a close companion star (center left; a sun-like main sequence or more evolved star). When the distance between two stars is close enough, the outer gas of the companion starts to accumulate on the surface of the white dwarf via an accretion disk. The thicker gas layer on the white dwarf increases its temperature and density. Then, nuclear reactions occur with a different way from those inside stars. In the case of stellar interiors, the huge energy produced by nuclear reactions in the core is balanced by the gravity of the surrounding gas, and then the reaction becomes stable. However, the nuclear reaction in a thin gas layer on the surface of a white dwarf has a different result. It becomes a runaway nuclear reaction, and results in an explosion that blows away the gas layer.
Two types of radial spins one vertical and one horizontal producing differentiated collection of mass proving the Eintstein’s reference planes:
After the explosion, the material in the region around the white dwarf is very hot and has a very high density. The light radiated by the white dwarf passes through some of the gaseous blobs blown away by the explosion, and that light reaches observers. Each element in the blobs absorbs the light at a specific wavelength. Because each blob has a different velocity (~1000 km/s), the nova spectrum shows many groups of weak absorption lines created by various elements.