Scientists have discovered a mutation in the Omicron BA.5 subvariant, allowing it to efficiently infect lung cells. This development could lead to more severe disease outcomes, especially in high-risk groups. The study emphasizes the importance of ongoing monitoring of Omicron subvariants’ evolution.
Future SARS-CoV-2 variants may also regain the ability to infect the lower respiratory tract.
Omicron-derived virus variants currently account for the majority of SARS-CoV-2 infections globally. Compared to its predecessors, Omicron generally leads to less severe disease. This is largely attributed to Omicron’s reduced ability to infect lung cells, and as a result, cause pneumonia less frequently. However, a team of international researchers, including scientists from the German Primate Center – Leibniz Institute for Primate Research, discovered a mutation in the spike protein of the Omicron subvariant BA.5. This mutation allows the virus to efficiently infect lung cells again.
This study provides evidence that Omicron subvariants may evolve in such a way that they regain their ability to effectively infect the lungs and cause severe illness in high-risk patients and individuals with inadequate immunity. The findings are published in the journal Nature Communications.
Omicron Subvariants: BA.1 and BA.2
The BA.1 and BA.2 Omicron subvariants dominated the COVID-19 pandemic during the first half of 2022. These subvariants, in comparison to the Delta variant and others, showed a reduced ability to infect lung cells. However, the infective efficiency of the BA.5 subvariant, which outcompeted other Omicron subvariants in the fall of 2022, was initially unclear. Markus Hoffmann and Stefan Pöhlmann from the German Primate Center led a team of researchers to show that, due to a mutation of the spike protein, BA.5 actually infects lung cells much more efficiently than previous Omicron subvariants.
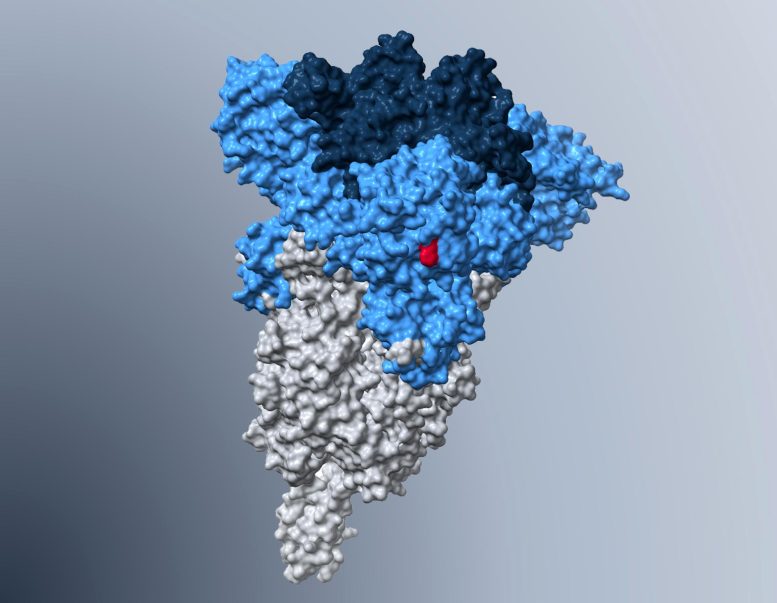
Model of the spike protein of the Omicron subvariant BA.5, in which the H69Δ/V70Δ mutation that is partly responsible for the increased lung cell entry is highlighted in red. Credit: Markus Hoffmann
Mutations in the Spike Protein and Their Impact
The researchers discovered that the spike protein of the BA.5 Omicron subvariant is cleaved more efficiently than its predecessors. Additionally, the spike protein of BA.5 facilitates the virus’s entry into lung cells and increases cell fusion efficiency. The team used “pseudo-viruses” as a safe model to examine how the virus penetrates lung cells.
Markus Hoffmann, the study’s first author, explains: “We found that BA.5 has acquired a mutation that allows the virus to penetrate lung cells more efficiently than the previously dominant Omicron subvariants. Thus, the ongoing evolution of Omicron subvariants may produce viruses in the future that efficiently spread into the lower respiratory tract and may cause severe disease, at least in patients without effective immune protection.” The altered properties of Omicron BA.5 are due to a key mutation known as “H69Δ/V70Δ.”

Infection biologist Dr. Markus Hoffmann (left) and Prof. Dr. Stefan Pöhlmann, head of the Infection Biology Unit at the German Primate Center (DPZ) – Leibniz Institute for Primate Research. Credit: Karin Tilch
Confirmation With Real Virus and Further Studies
To validate these findings, Christian Drosten’s team at the Virology Department of Charité – Berlin University Hospital performed additional experiments with real viruses. These tests confirmed that BA.5 strain viruses infect lung cells efficiently, corroborating the results from Göttingen. To determine whether Omicron BA.5 also infects lung cells in living organisms, researchers at the University of Iowa compared the lungs of mice that were infected with BA.5 with those that received other subvariants. They found that BA.5 replicated up to 1000 times more efficiently in the lungs of mice compared to earlier Omicron subvariants.
Lastly, experiments conducted on ferrets at the Friedrich-Loeffler-Institut in Greifswald – Insel Riems, Germany, revealed that the BA.5 subvariant spreads more efficiently in the upper respiratory tract than previous virus variants.
“Altogether, this suggests that similarly to other Omicron subvariants, BA.5 is highly contagious and has additionally evolved the ability to efficiently infect lung cells,” says Stefan Pöhlmann, head of the Infection Biology Unit at the German Primate Center. “The further evolution of Omicron subvariants should therefore be monitored closely in order to be able to quickly identify variants with increased risk potential.”
Reference: “Omicron subvariant BA.5 efficiently infects lung cells” by Markus Hoffmann, Lok-Yin Roy Wong, Prerna Arora, Lu Zhang, Cheila Rocha, Abby Odle, Inga Nehlmeier, Amy Kempf, Anja Richter, Nico Joel Halwe, Jacob Schön, Lorenz Ulrich, Donata Hoffmann, Martin Beer, Christian Drosten, Stanley Perlman and Stefan Pöhlmann, 13 June 2023, Nature Communications.
DOI: 10.1038/s41467-023-39147-4


Be the first to comment on "Omicron Mutation: How the Omicron Subvariant Evolved To Breach Lung Defenses"