
New research has uncovered the importance of atomic ring structures in glass, revealing how their stability influences glass’s performance and transition temperatures. This advance in understanding glass’s molecular dynamics aids in designing better glass products for high-performance applications.
Glass is increasingly utilized in various high-performance areas, covering consumer and industrial applications, military and aerospace electronics, as well as coatings and optics. Given the stringent accuracy required in products like mobile phones and jet aircraft, it’s crucial that glass substrates remain unaltered in shape throughout the manufacturing process.
Corning Incorporated, a manufacturer of innovative glass, ceramics, and related materials, invests a tremendous amount of resources into studying the stability of different types of glass. Recently, Corning researchers found that understanding the stability of the rings of atoms in glass materials can help them predict the performance of glass products. This capability is important because the most widely used glass is silicate glass, which consists of different sizes of atomic rings connected in three dimensions.
Neutron Scattering Experiments
Conducting neutron scattering experiments at the Department of Energy’s Oak Ridge National Laboratory, ORNL and Corning scientists discovered that as the number of smaller, less-stable atomic rings in a glass increases, the instability, or liquid fragility, of the glass also increases. The results of the neutron experiments, published in Nature Communications, reveal a clear correlation between the medium-range atomic ring structure of a silicate glass and its liquid fragility. The viscosity of the liquid glass changes considerably when it is cooled to the glass transition temperature. A more fragile liquid will have a larger viscosity change with a given temperature change.
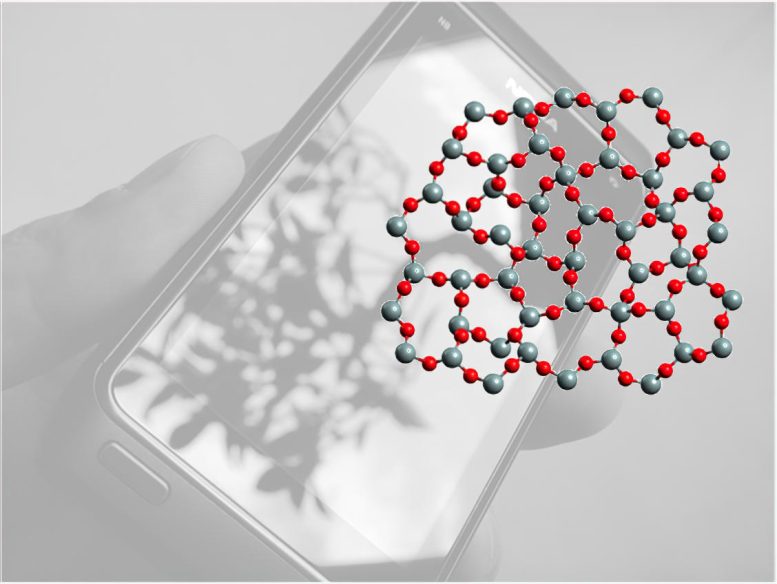
Oak Ridge National Laboratory and Corning scientists discovered that as the number of smaller, less-stable atomic rings in a glass increases, the instability, or liquid fragility, of the glass also increases. Credit: Titanas from Athens, Greece
“Previously, the mechanism driving glass transitions had eluded scientists,” said Ying Shi, the corresponding author of the study’s paper and research associate at Corning. “There was no clear understanding as to why some types of glass solidified faster or slower.”
Shi and her collaborators from Corning, the University of California, Los Angeles, and the University of Oxford worked with the NOMAD neutron diffractometer beamline scientists at ORNL’s Spallation Neutron Source to study aluminosilicate glass, which is commonly used by industry.
Development of RingFSDP
Using a recently developed and validated neutron scattering data analysis tool, RingFSDP, the team identified key patterns in the collected data that revealed the relationship between the liquid fragility in the glass and its atomic ring stability.
RingFSDP is a free, open-source program developed by Corning and ORNL scientists to study the atomic ring structures of silicate glass. It derives ring-size distributions in silicate glass from the shape of the first sharp diffraction peak in the neutron diffraction data.
“Connecting the glass transition temperature range to underlying structural features of a glass will have a significant impact on glass design and production,” said Douglas Allan, the paper’s co-author and a research fellow at Corning. “Our work shows a clear correlation between the atomic ring structure of a glass and its glass transition temperature range and therefore the performance features of the glass.”
Reference: “Revealing the relationship between liquid fragility and medium-range order in silicate glasses” by Ying Shi, Binghui Deng, Ozgur Gulbiten, Mathieu Bauchy, Qi Zhou, Jörg Neuefeind, Stephen R. Elliott, Nicholas J. Smith and Douglas C. Allan, 3 January 2023, Nature Communications.
DOI: 10.1038/s41467-022-35711-6
The study included researchers from the Science and Technology Division at Corning, the Physics of Amorphous and Inorganic Solids Laboratory in the Department of Civil and Environmental Engineering at the University of California, Los Angeles, the Neutron Scattering Division at ORNL’s SNS and the Physical and Theoretical Chemistry Laboratory at the University of Oxford.








Be the first to comment on "Reinventing Glass: A Breakthrough in Atomic Stability"