Stanford University researchers are developing an ultrafast insulin formulation that could be four times faster than current fast-acting insulin options.
Researchers at Stanford University are developing a new insulin formulation that begins to take effect almost immediately upon injection, potentially working four times as fast as current commercial fast-acting insulin formulations.
The researchers focused on so-called monomeric insulin, which has a molecular structure that, according to theory, should allow it to act faster than other forms of insulin. The catch is that monomeric insulin is too unstable for practical use. So, in order to realize the ultrafast potential of this insulin, the researchers relied on some materials science magic.
“The insulin molecules themselves are fine, so we wanted to develop a ‘magic fairy dust’ that you add into a vial that would help to fix the stability problem,” said Eric Appel, assistant professor of materials science and engineering at Stanford. “People often focus on the therapeutic agents in a drug formulation but, by focusing only on the performance additives — parts that were once referred to as ‘inactive ingredients’ — we can achieve really big advancements in the overall efficacy of the drug.”
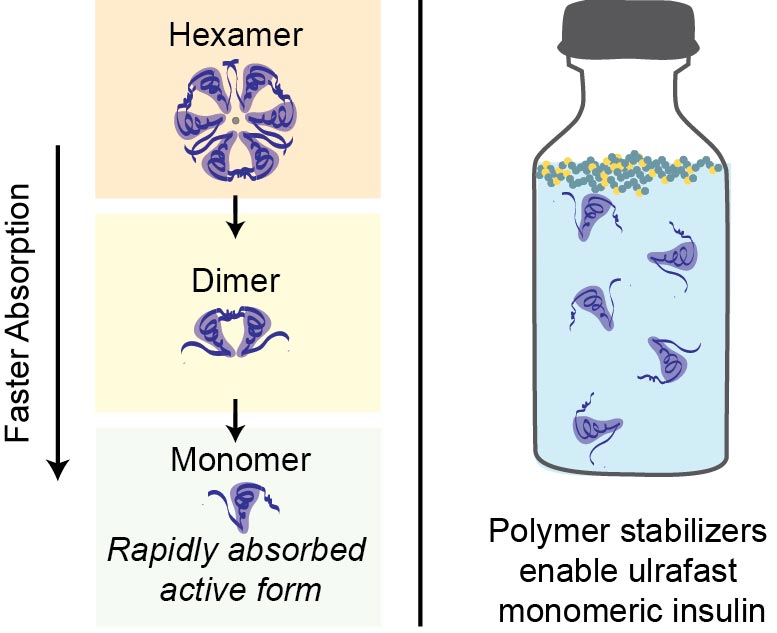
Illustration depicting how fast different forms of insulin absorb in the bloodstream, and how the polymer developed by these researchers helps stabilize ultrafast-absorbing insulin in the vial. Credit: Joseph Mann and Caitlin Maikawa
After screening and testing a large library of additive polymers, the researchers found one that could stabilize monomeric insulin for more than 24 hours in stressed conditions. (By comparison, commercial fast-acting insulin stays stable for six to ten hours under the same conditions.) The researchers then confirmed the ultrafast action of their formulation in diabetic pigs. Their results were published today (July 1, 2020) in Science Translational Medicine. Now, the researchers are conducting additional tests in hopes of qualifying for clinical trials in humans.
One step back, two steps forward
Current commercial formulations of insulin contain a mix of three forms: monomers, dimers, and hexamers. Scientists have assumed monomers would be the most readily useful in the body but, within vials, the insulin molecules are drawn to the surface of the liquid where they aggregate and become inactive. (Hexamers are more stable in the vial but take longer to work in the body because they first have to break down into monomers to become active.) This is where the “magic fairy dust” — a custom polymer that is attracted to the air/water interface — comes in.
“We focused on polymers that would preferentially go to that interface and act as a barrier between any of the insulin molecules trying to gather there,” said Joseph Mann, a graduate student in the Appel lab and co-lead author of the paper. Crucially, the polymer can do this without interacting with the insulin molecules themselves, allowing the drug to take effect unimpeded.
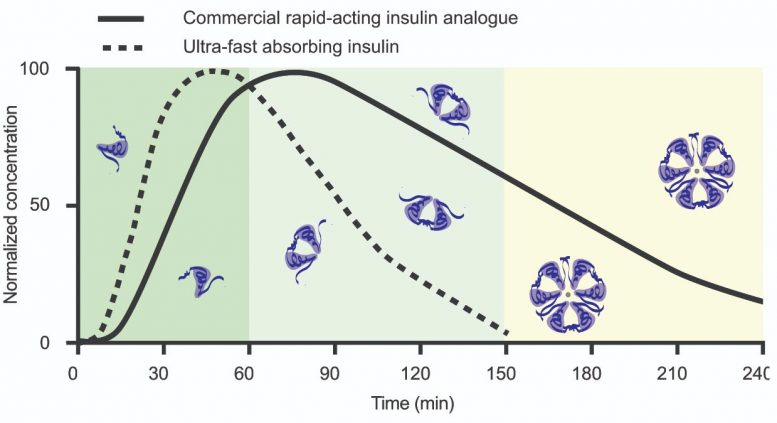
The ultrafast-absorbing insulin is based on simpler insulin monomer molecules, which are absorbed far faster than the more complex dimers and hexamers used in commercial rapid-acting insulin analogs. This material relates to a paper that appeared in the Jul. 1, 2020, issue of Science Translational Medicine, published by AAAS. The paper, by J.L. Mann at Stanford University in Stanford, CA; and colleagues was titled, “An ultrafast insulin formulation enabled by high-throughput screening of engineered polymeric excipients.” Credit: J.L. Mann et al., Science Translational Medicine (2020)
Finding just the right polymer with the desired properties was a long process that involved a three-week trip to Australia, where a fast-moving robot created approximately 1500 preliminary candidates. This was followed by processing and testing individually by hand at Stanford to identify polymers that successfully exhibited the desired barrier behavior. The first 100 candidates didn’t stabilize commercial insulin in tests but the researchers pressed on. They found their magic polymer only weeks before they were scheduled to run experiments with diabetic pigs.
“It felt like there was nothing happening and then all of a sudden there was this bright moment … and a deadline a couple of months away,” said Mann. “The moment we got an encouraging result, we had to hit the ground running.”
In commercial insulin — which typically remains stable for about 10 hours in accelerated aging tests — the polymer drastically increased the duration of stability for upwards of a month. The next step was to see how the polymer affected monomeric insulin, which on its own aggregates in 1-2 hours. It was another welcome victory when the researchers confirmed that their formulation could remain stable for over 24 hours under stress.
“In terms of stability, we took a big step backward by making the insulin monomeric. Then, by adding our polymer, we met more than double the stability of the current commercial standard,” said Caitlin Maikawa, a graduate student in the Appel lab and co-lead author of the paper.
With a seed grant from the Stanford Diabetes Research Center and the Stanford Maternal and Child Health Research Institute, the researchers were able to evaluate their new monomeric insulin formulation in diabetic pigs — the most advanced non-human animal model — and found that their insulin reached 90 percent of its peak activity within five minutes after the insulin injection. For comparison, the commercial fast-acting insulin began showing significant activity only after 10 minutes. Furthermore, the monomeric insulin activity peaked at about 10 minutes while the commercial insulin required 25 minutes. In humans, this difference could translate to a four-fold decrease in the time insulin takes to reach peak activity.
“When I ran the blood tests and started plotting the data, I almost couldn’t believe how good it looked,” said Maikawa.
“It’s really unprecedented,” said Appel, who is senior author of the paper. “This has been a major target for many big pharmaceutical companies for decades.”
The monomeric insulin also finished its action sooner. Both beginning and ending activity sooner makes it easier for people to use insulin in coordination with mealtime glucose levels to appropriately manage their blood sugar levels.
A multifaceted success
The researchers plan to apply to the Food and Drug Administration for approval to test their insulin formulation in clinical trials with human participants (although no trials are planned yet and they are not seeking participants at this time). They are also considering other uses for their polymer, given how significantly it increased stability in commercial insulin.
Because their insulin formulation activates so quickly — and, therefore, more like insulin in a person without diabetes — the researchers are excited by the possibility that it could aid the development of an artificial pancreas device that functions without the need for patient intervention at mealtimes.
Reference: “An ultrafast insulin formulation enabled by high-throughput screening of engineered polymeric excipients” by Joseph L. Mann, Caitlin L. Maikawa, Anton A. A. Smith, Abigail K. Grosskopf, Sam W. Baker, Gillie A. Roth, Catherine M. Meis, Emily C. Gale, Celine S. Liong, Santiago Correa, Doreen Chan, Lyndsay M. Stapleton, Anthony C. Yu, Ben Muir, Shaun Howard, Almar Postma and Eric A. Appel, 1 July 2020, Science Translational Medicine.
DOI: 10.1126/scitranslmed.aba6676
Additional Stanford co-authors include former visiting scholar Anton Smith (from Aarhus University in Denmark); graduate students Abigail Grosskopf, Gillie Roth, Catherine Meis, Emily Gale, Celine Liong, Doreen Chan, Lyndsay Stapleton and Anthony Yu; clinical veterinarian Sam Baker; and postdoctoral fellow Santiago Correa. Researchers from CSIRO Manufacturing in Australia are also co-authors. Appel is also a member of Stanford Bio-X, the Cardiovascular Institute, the Stanford Maternal and Child Research Institute and a faculty fellow at Stanford ChEM-H.
This research was funded by the National Institutes of Health, a Pilot and Feasibility seed grant from the Stanford Diabetes Research Center, the Stanford Maternal and Child Health Research Institute, the American Diabetes Association, the PhRMA Foundation, the U.S. Department of Defense, a Stanford Graduate Fellowship, the Natural Sciences and Engineering Research Council of Canada, the Stanford Bio-X Bowes Graduate Student Fellowship, the Novo Nordisk Foundation, Stanford Bio-X, and the Danish Council of Independent Research.






Oh,yes!! Ive been type 1 diabetic for 31 years now. I can’t wait to pay $10,000 a month for this awesome new drug…….
If anyone is interested in a slightly faster working insulin, look into Fiasp. It’s fairly new on the market, but I’ve been on it for a few months and it does begin to work faster than Novolog and Humalog. It seems to begin working 20 minutes faster than those insulins I mentioned.
I am a type 1 diabetic for 61 years, and have insulin resistance. It takes almost 4 hour for humalog to work so eating is planned way in advance. It would be great if this insulin cut the time lag to 2 hours.
I seriously doubt this will be widely released, but it could save the lives of people with severe diabetic complications such as ketoacidosis that need an immediate shot of insulin.
Fiasp, don’t know what it stands for but I call it Fast Insulin As Soon as Possible! So much better than Apidra, Novolog and Humalog. Tired of your so called 15 minute insulin taking 4 hours, try Fiasp. Actually works for me.
It’s not going to solve much as long as there is no cure for diabetes…..it is the greed of the pharmaceutical companies that is preventing cure for diabetes and othe diseases.
I have found my own method to make fast-acting insuoin work very quickly. If you combine the taking of insulin with exercise, the insulin works very quickly. You can reduce a 280 blood sugar level to 100 within a short amount of time. Needless to say, you have to be cautious and monitor your blood sugar while this is taking place, lest you become hypoglycemic.
How exciting! Is Joseph Mann by any chance related to Al Mann the inventor of Inhaled Insulin? http://www.inhalemyinsulin.com
Afreeza.
If the issue with stability is air in the vial, instead of adding polymer why not just make the vial collapsible. Airtight. Maybe smaller quantities per container. Lots of physical options. Like the injection pens, but without the air bubble.
My Son is a Type 1 diabetic since he was 3 yrs and now he is 22yrs .since last 19 yrs he is insulin dependant 4 times daily. All these big Organization,can you first research and develop a hand watch that can show readings realtime please .The drug can come subsequently
I am type 2 Diabetics patient, I am interested to purchase. How can I get it to get delivery in Goma, DR Congo, East Africa.
Am diabetes type 2 please am interested
Type 2 diabetes can save the money. There is no insulin needed. Just 0 carb. Not low carb really 0. No fruit juice, no soft drinks, no bread, no noodles and no rice. Important, no processed food. And combine with just 2 or 1 meal a day. you will be healthy in no time, not hungry and if you are fat, this will disappear also. I know what I talk about. If your doctor says different, may be considder changing the doctor and the food together. I have lots of references of MD’s and scientists where you can learn from.
what about potatoes?
Wow they made injectable Afrezza. This new insulin would definitely be useful for pump users but if you want all theses benefits TODAY ask your doctor for Afrezza. FDA approved for years and no needles.
this is a giant step in the right direction however, developing countries suffer the most because it takes ages to get the latest drugs/medicine. I am also interested to know the type of food that i can eat to keep in control diabetes in my body.
I like what Georg krause has shared regarding food items. Please sir i need more on these important foods that one can eat to keep diabetes at bay- over to you Georg keep in touch through my mail.
Hi thanks
Mannkind holds the patent on human monomeric insulin. When added to fdkp it stabilizes and can be inhaled which is faster than FAISP in and out and kuess than 30 seconds difference from the healthy pancreas..but also the patent also covers Monomeric insulin on technosphere in a saline solution as well so it’s already under patent..by Mannkind..but inhalable is there fastest systemic delivery Afrezza..fda approved in 2014..
Afrezza is Less than 30 seconds difference from the healthy pancreas..you also need an edit option for comments
Inhaled insulin, like Afrezza, seems like a poor choice during a virus pandemic which targets the respiratory system.
How will Mannkind’s patent on monomeric insulin in saline solution factor on development of this new product? Isn’t the patent on the process rather than the actual substance? Need a patent lawyer to fill in the details here.
Actually Jad..there is a user of Afrezza that had Covid-19 (see MNKD Proboards) and never had to go to the ICU and is doing fine! With so many diabetics that die from Covid-19 and the fact that insulin is an anti-inflammitory..someone should study Afrezza’s for Covid-19 to see if other users have simular outcomes..verses non users and other insulin types…it maybe that switching over to Afrezza..that does go to the lungs may actually not be such a “poor choice” after all..(again..if studied and if results of outcomes demonstrate such things) hmm (long MNKD)
I was on 2 medications which were constantly being increased and the side effects were awful. This nutritional plan got my pancreas working normally again and reversed my diabetes: http://www.diabetes14.com