
A team of researchers has developed a new research paradigm which simplifies the understanding of how catalyst structures affect their reactions. The study focused on the electrochemical CO2 reduction reaction using Tin-Oxide-based catalysts, revealing crucial insights into the active surface species and their performance. This breakthrough allows for the tailored design of efficient catalysts and paves the way for further exploration of electrocatalytic reactions, aiming to enhance the development of scalable and high-performance electrocatalysts.
In a significant advance in the fight against climate change and the shift towards sustainability, a team of researchers has introduced a new research framework that simplifies understanding how catalyst structures influence their reactions.
Details of the researchers’ breakthrough were published in the journal Angewandte Chemie.
Understanding how a catalyst’s surface affects its activity can aid the design of efficient catalyst structures for specific reactivity requirements. However, grasping the mechanisms behind this relationship is no straightforward task given the complicated interface microenvironment of electrocatalysts.
“To decipher this, we honed in on the electrochemical CO2 reduction reaction (CO2RR) in Tin-Oxide-based (Sn-O) catalysts,” points out Hao Li, associate professor at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR) and corresponding author of the paper. “In doing so, we not only uncovered the active surface species of SnO2-based catalysts during CO2RR but also established a clear correlation between surface speciation and CO2RR performance.”
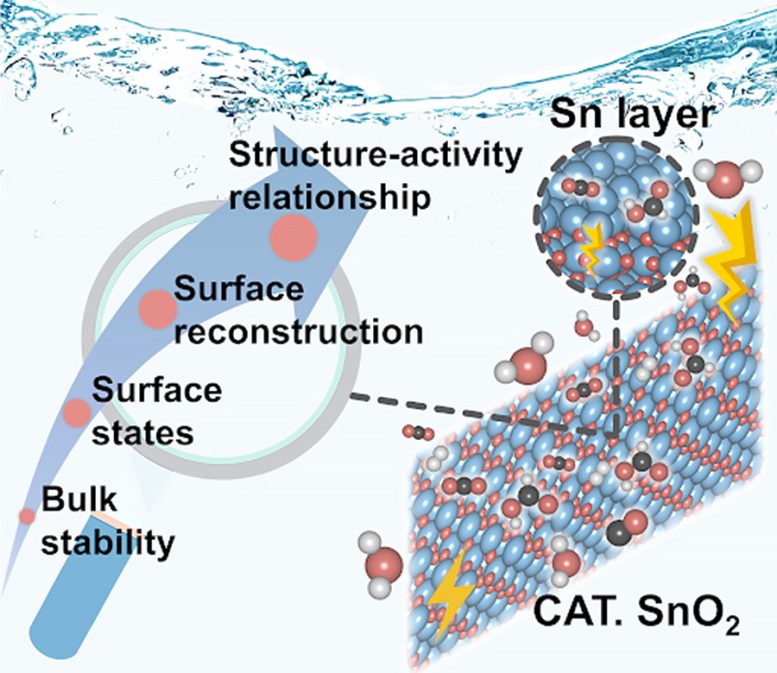
The standard research paradigm uncovers the structure-property-activity relationships for the electrochemical CO2 reduction reaction (CO2RR) over SnO2. This picture illustrates the surface reconstruction induced by oxygen vacancies (1/1 ML coverage) and surface-active species (Sn layer) accountable for selective HCOOH production. Credit: Hao Li et al.
Promising Method for CO2 Reduction
CO2RR is recognized as a promising method for reducing CO2 emissions and producing high-value fuels, with formic acid (HCOOH) being a noteworthy product because of its various applications in industries such as pharmaceuticals, metallurgy, and environmental remediation.
The proposed method helped identify the genuine surface states of SnO2 responsible for its performance in CO2 reduction reactions under specific electrocatalytic conditions. Moreover, the team corroborated their findings through experiments using various SnO2 shapes and advanced characterization techniques.
Li and his colleagues developed their methodology by combining theoretical studies with experimental electrochemical techniques.
“We bridged the gap between the theoretical and experimental, offering a comprehensive understanding of catalyst behavior under real-world conditions in the process,” adds Li.
The research team is now focused on applying this methodology to a variety of electrochemical reactions. In doing those, they hope to uncover more about unique structure-activity correlations, accelerating the design of high-performance and scalable electrocatalysts.
Reference: “Deciphering Structure-Activity Relationship Towards CO2 Electroreduction over SnO2 by A Standard Research Paradigm” by Zhongyuan Guo, Yihong Yu, Congcong Li, Egon Campos dos Santos, Tianyi Wang, Huihui Li, Jiang Xu, Chuangwei Liu and Hao Li, 29 January 2024, Angewandte Chemie International Edition.
DOI: 10.1002/anie.202319913






Be the first to comment on "Revolutionizing Catalyst Design: New Research Links Structure to Reaction Performance"