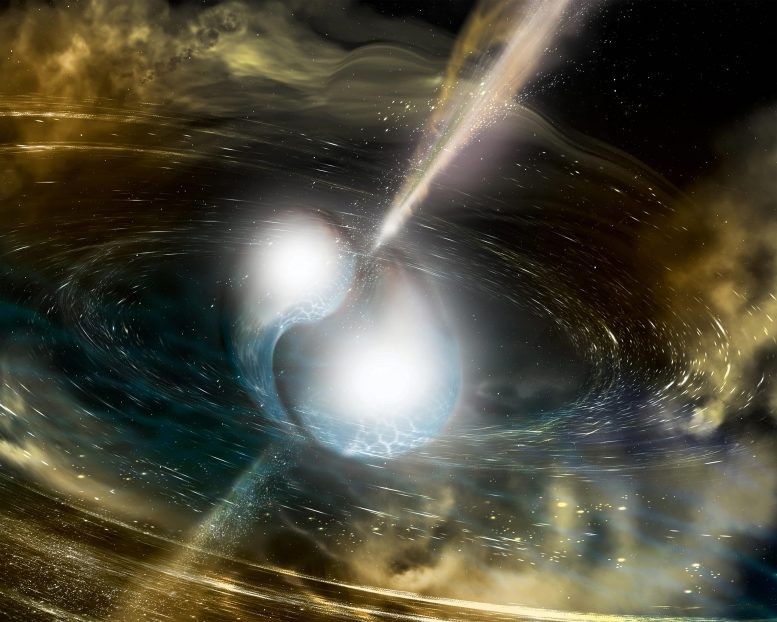
An artist’s depiction of a neutron star merger. These mergers produce heavy elements like gold and platinum in abundance. Credit: Sonoma State University/A. Simonnet
What Is Nucleosynthesis?
Nucleosynthesis is the creation of new atomic nuclei, the centers of atoms that are made up of protons and neutrons. Nucleosynthesis first occurred within a few minutes of the Big Bang. At that time, a quark-gluon plasma, a soup of particles known as quarks and gluons, condensed into protons and neutrons. After the universe cooled slightly, the neutrons fused with protons to make nuclei of deuterium, an isotope of hydrogen. Deuterium nuclei then combined to make helium. Further reactions between protons, neutrons, and different isotopes of helium produced lithium. The hydrogen and helium produced during this phase of the universe eventually created the universe’s first massive stars.
Since then, the nuclear reactions in the life and death of stars have formed most of the other nuclei in the universe. Stars can create nuclei through two processes: either by combining two smaller nuclei (called fusion) or breaking a larger nucleus into multiple nuclei (called fission). Both ways result in new atoms.
In the past, these processes also produced the elements on the Periodic Table that we know today. Stars of different types produce nuclei of different elements, leading over time to the range of natural elements. The universe’s first stars were massive, often more than 10 times the size of our Sun. They also had far shorter lives than stars that existed more recently. As they lived, they burned hydrogen and produced the elements up to iron in the Periodic Table. When they died, they ejected nuclei of these elements in a type of explosion called a core-collapse supernova. Supernovae can leave behind neutron stars. When neutron stars merge, they produce new nuclei, including elements that are heavier than iron. Other stars become white dwarfs as they die. These white dwarfs may also later merge and synthesize nuclei of elements.
Nucleosynthesis Facts
- Scientists believe the heaviest naturally occurring elements, including uranium, are produced in violent neutron-rich environments such as the merger of two neutron stars or supernovae. Under these conditions, neutrons gain nuclei faster than they can decay.
- We consist mostly of matter created through nucleosynthesis in stars that have since died, leading to cosmologist Carl Sagan’s famous statement that we are made of “star-stuff.”
DOE Office of Science: Nucleosynthesis Contributions
The Office of Nuclear Physics in the DOE Office of Science supports research in nuclear astrophysics—the physics needed to understand the reactions that produce the elements. Two university-based DOE Centers of Excellence, the Cyclotron Institute at Texas A&M University and the Triangle Universities Nuclear Laboratory, specialize in the study of nuclear astrophysics. DOE also funds the theory and modeling of the Big Bang, stars, supernovae, and neutron star mergers, all sources of elements.
The DOE Office of Science’s Argonne Tandem Linac Accelerator System (ATLAS) user facility is home to the world’s most powerful spectrometer for nuclear structure research. Moving forward, the Office of Nuclear Physics is now supporting the construction of the Facility for Rare Isotope Beams at Michigan State University. This accelerator will produce short-lived and never-before-seen neutron-rich nuclei that play a role in the production of the heaviest elements.







Nice concise article. One correction, in the R process, “nuclei gain neutrons faster than they can decay” not the other way around.
It would have been useful to include the graphic periodic table of elements by nucleosynthesis too, preferably the wide version, it labels each element with 6 colors by how it was made.
Also not long after any super nova or collision event, all ejected neutrons will decay back to protons & electrons as cosmic radiation if they didn’t get used in synthesis.
Also the earliest stars may have been 10x or more times the size of the Sun but they still had to use the slower pp chain to build helium in the 3 step process (see wikipedia). As soon as carbon became present in the latter stage of their life, then things can speed up with the CNO cycle which runs 10,000 times faster. I would speculate that the 3 step slow pp cycle could also be sped up if the the 1st constraint to convert proton pairs into neutrons is simply skipped by using up any available big bang deuterium already available, making it possible to make helium in 2 steps and only add more deuterium making as the star matures. I’d guess that in the 1st moments of star ignition, only the latter 2 steps are needed converting BB deuterium directly to helium 3 then helium 4. If the big bang had never made deuterium, I’d bet there would be no stars formed as we know them.
… it would be a very interesting to create a graph that would represent all possible mutations of elementary particles…
… by the way, why is proton that stable? Is there an Entropy at that level! Or it is just an obvious example of our humanity?…