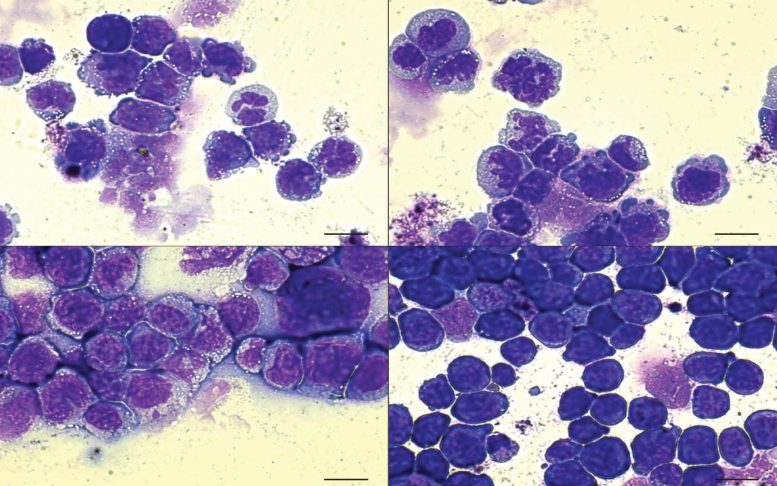
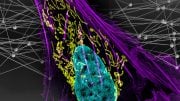
In AML, defective blood cells (stained purple) are present. When the cells have a double mutation of both the IDH2 and SRSF2 genes (bottom right), the number of defective cells rises considerably, indicating a more lethal disease. Credit: Krainer lab/CSHL, 2019
The whole is sometimes bigger than the sum of its parts. Cold Spring Harbor Laboratory researchers revealed that two cell abnormalities, which are already damaging on their own, boost each other’s effects, contributing to the development of the deadly blood malignancy acute myeloid leukemia (AML).
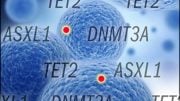
CSHL Professor Adrian Krainer and his lab, along with Omar Abdel-Wahab at Memorial Sloan Kettering Cancer Center, detailed how mutations of the genes IDH2 and SRSF2 are unexpected partners-in-crime for causing AML.
The presence of the IDH2 mutation, in particular, exacerbates the defects induced by the SRSF2 mutation, preventing cells in the bone marrow from growing into the red and white blood cells required for an AML patient to fight the disease. The team is currently looking for ways to rapidly terminate this collaboration, resulting in a strong treatment for blood cancer.
“This all started when we were looking at patient data in The Cancer Genome Atlas and saw that again and again deadly cases of AML had both these mutations,” said Abdel-Wahab, a hematologist oncologist.
Prior to this study, the only known connection between the two mutations was that they both cause pre-cancerous signs. However, in many circumstances, the source of the symptoms is not the same as the cause of the cancer itself.
“Just because you see a mutation [in a sick patient’s cells] doesn’t really show that it’s directly contributing to the disease,” Krainer said.
To determine if the IDH2 and SRSF2 mutations are indeed at work in AML, Abdel-Wahab joined forces with Krainer’s lab. The team detailed their findings in a paper recently published in Nature.
The researchers knew that one of the two mutations in question, the SRSF2 gene, causes errors in a crucial process called RNA splicing. Splicing converts messages from DNA, called RNA, into readable instructions for a cell. Errors in this process can result in serious cell malfunction.
Initially, researchers did not believe SRSF2-driven splicing problems were linked to AML since DNA tests revealed the abnormalities in just 1% of AML patients. The Krainer lab, which specializes in RNA splicing, discovered that this issue is far more widespread, occurring around 11% of the time in AML patients.
Kuan-Ting Lin, a postdoctoral researcher from Krainer’s lab, discovered this by searching for signs of the SRSF2 mutation in RNA. Instead of merely exploring an entire office building, his search may be compared to dusting for SRSF2’s fingerprints within the gene’s actual workplace (RNA) (DNA).
“Dr. Lin is very patient, looking at massive amounts of data and picking up distinct patterns and making connections between things that aren’t obvious to everyone,” Krainer said.
Further experiments from Abdel-Wahab’s lab revealed that the severity of the identified SRSF2 splicing errors can be enhanced by the presence of a second mutation, IDH2, resulting in even more defective blood cells.
“So in some ways, these two genes, when defective, are cooperating,” Krainer said. “Now that we do know about this interdependence, we may find points where we can intervene.”
###
This research was supported in part by the Aplastic Anemia and MDS International Foundation and the Lauri Strauss Leukemia Foundation, as well as Cancer Research UK and the Welch Foundation. Additional support comes from the NIH/NHLBI, the Leukemia and Lymphoma Society, The Oglesby Charitable Trust, the Dept. of Defense Bone Marrow Failure Research Program, the Starr Foundation, the Henry & Marilyn Taub Foundation, the Edward P. Evans Foundation, the Josie Robertson Investigator Program, the Leukemia and Lymphoma Society, and the Pershing Square Sohn Cancer Research Alliance.
Reference: “Coordinated alterations in RNA splicing and epigenetic regulation drive leukaemogenesis” by Akihide Yoshimi, Kuan-Ting Lin, Daniel H. Wiseman, Mohammad Alinoor Rahman, Alessandro Pastore, Bo Wang, Stanley Chun-Wei Lee, Jean-Baptiste Micol, Xiao Jing Zhang, Stephane de Botton, Virginie Penard-Lacronique, Eytan M. Stein, Hana Cho, Rachel E. Miles, Daichi Inoue, Todd R. Albrecht, Tim C. P. Somervaille, Kiran Batta, Fabio Amaral, Fabrizio Simeoni, Deepti P. Wilks, Catherine Cargo, Andrew M. Intlekofer, Ross L. Levine, Heidi Dvinge, Robert K. Bradley, Eric J. Wagner, Adrian R. Krainer and Omar Abdel-Wahab, 2 October 2019, Nature.
DOI: 10.1038/s41586-019-1618-0




Be the first to comment on "When Mutant Cells Team Up, an Even Deadlier Blood Cancer Emerges [Video]"