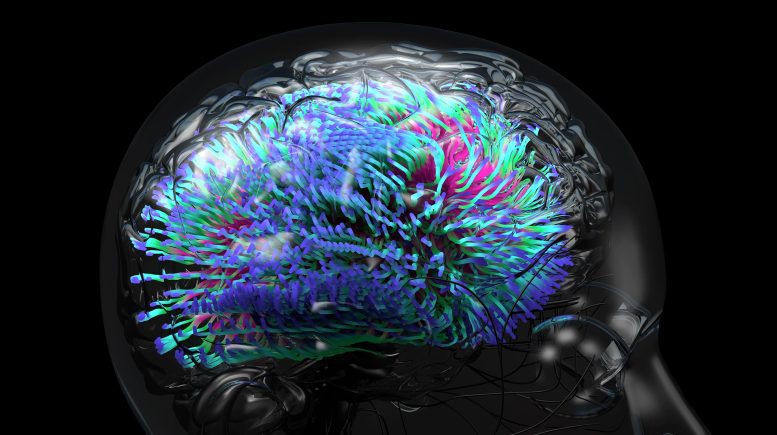
UCLA scientists have found a new genetic risk factor for Alzheimer’s disease by screening people’s DNA and then using an advanced type of scan to visualize their brains’ connections.
Using a new imaging method that screens the brain’s connections, UCLA scientists have discovered a common abnormality in our genetic code that increases the risk of Alzheimer’s years before the disease strikes.
Scientists at the University of California, Los Angeles (UCLA) have discovered a new genetic risk factor for Alzheimer’s disease by screening people’s DNA and then using an advanced type of scan to visualize their brains’ connections.
Alzheimer’s disease, the most common cause of dementia in the elderly, erodes these connections, which we rely on to support thinking, emotion and memory. With no known cure for the disease, the 20 million Alzheimer’s sufferers worldwide lack an effective treatment. And we are all at risk: Our chance of developing Alzheimer’s doubles every five years after age 65.
The UCLA researchers discovered a common abnormality in our genetic code that increases the risk of Alzheimer’s. To find the gene, they used a new imaging method that screens the brain’s connections — the wiring, or circuitry, that communicates information. Switching off such Alzheimer’s risk genes (nine of them have been implicated over the last 20 years) could stop the disorder in its tracks or delay its onset by many years.

Paul Thompson. Credit: UCLA
The research is published in the March 4 online edition of the journal Proceedings of the National Academy of Sciences.
“We found a change in our genetic code that boosts our risk for Alzheimer’s disease,” said the study’s senior author, Paul Thompson, a UCLA professor of neurology and a member of the UCLA Laboratory of Neuro Imaging. “If you have this variant in your DNA, your brain connections are weaker. As you get older, faulty brain connections increase your risk of dementia.”
The researchers, Thompson said, screened more than a thousand people’s DNA to find the common “spelling errors” in the genetic code that might heighten their risk for the disease later in life. The new study was the first of its kind to also give each person a “connectome scan,” a special type of scan that measures water diffusion in the brain, allowing scientists to map the strength of the brain’s connections.
The new scan reveals the brain’s circuitry and how information is routed around the brain, in order to discover risk factors for disease. The researchers then combined these connectivity scans with the extensive genomic screening to pinpoint what causes faulty wiring in the brain.
Hundreds of computers, calculating for months, sifted through more than 4,000 brain connections and the entire genetic code, comparing connection patterns in people with different genetic variations. In people whose genetic code differed in one specific gene called SPON1, weaker connections were found between brain centers controlling reasoning and emotion. The rogue gene also affects how senile plaques build up in the brain — one of the hallmarks of Alzheimer’s disease.
“Much of your risk for disease is written in your DNA, so the genome is a good place to look for new drug targets,” said Thompson, who in 2009 founded a research network known as Project ENIGMA to pool brain scans and DNA from 26,000 people worldwide. “If we scan your brain and DNA today, we can discover dangerous genes that will undermine your ability to think and plan and will make you ill in the future. If we find these genes now, there is a better chance of new drugs that can switch them off before you or your family get ill.”
Developing new therapeutics for Alzheimer’s is a hot area for pharmaceutical research, Thompson said.
It has also been found that the SPON1 gene can be manipulated to develop new treatments for the devastating disease, Thompson noted. When the rogue gene was altered in mice, it led to cognitive improvements and fewer plaques building up in the brain. Alzheimer’s patients show an accumulation of these senile plaques, which are made of a sticky substance called amyloid and are thought to kill brain cells, causing irreversible memory loss and personality changes.
Screening genomes has led to many new drug targets in the treatment of cancer, heart disease, arthritis and brain disorders such as epilepsy. But the UCLA team’s approach — screening genomes and performing brain scans of the same people — promises a faster and more efficient search.
“With a brain scan that takes half an hour and a DNA scan from a saliva sample, we can search your genes for factors that help or harm your brain’s connections,” Thompson said. “This opens up a new landscape of discovery in medical science.”
Publication: Neda Jahanshad, et al., “Genome-wide scan of healthy human connectome discovers SPON1 gene variant influencing dementia severity” by Neda Jahanshad, Priya Rajagopalan, Xue Hua, Derrek P. Hibar, Talia M. Nir, Arthur W. Toga, Clifford R. Jack, Jr, Andrew J. Saykin, Robert C. Green, Michael W. Weiner, Sarah E. Medland, Grant W. Montgomery, Narelle K. Hansell, Katie L. McMahon, Greig I. de Zubicaray, Nicholas G. Martin, Margaret J. Wright, Paul M. Thompson, the Alzheimer’s Disease Neuroimaging Initiative, Michael Weiner, MD, Paul Aisen, MD, Michael Weiner, MD, Paul Aisen, MD, Ronald Petersen, MD, PhD, Clifford R. Jack, Jr., MD, William Jagust, MD, John Q. Trojanowski, MD, PhD, Arthur W. Toga, PhD, Laurel Beckett, PhD, Robert C. Green, MD, MPH, Andrew J. Saykin, PsyD, John Morris, MD, Enchi Liu, PhD, Robert C. Green, MD, MPH, Tom Montine, MD, PhD, Ronald Petersen, MD, PhD, Paul Aisen, MD, Anthony Gamst, PhD, Ronald G. Thomas, PhD, Michael Donohue, PhD, Sarah Walter, MSc, Devon Gessert, Tamie Sather, Laurel Beckett, PhD, Danielle Harvey, PhD, Anthony Gamst, PhD, Michael Donohue, PhD, John Kornak, PhD, Clifford R. Jack, Jr., MD, Anders Dale, PhD, Matthew Bernstein, PhD, Joel Felmlee, PhD, Nick Fox, MD, Paul Thompson, PhD, Norbert Schuff, PhD, Gene Alexander, PhD, Charles DeCarli, MD, William Jagust, MD, Dan Bandy, MS, CNMT, Robert A. Koeppe, PhD, Norm Foster, MD, Eric M. Reiman, MD, Kewei Chen, PhD, Chet Mathis, MD, John Morris, MD, Nigel J. Cairns, PhD, Lisa Taylor-Reinwald, BA, J.Q. Trojanowki, MD, PhD, Les Shaw, PhD, Virginia M.Y. Lee, PhD, MBA, Magdalena Korecka, PhD, Arthur W. Toga, PhD, Karen Crawford, Scott Neu, PhD, Andrew J. Saykin, PsyD, Tatiana M. Foroud, PhD, Steven Potkin, MD, Li Shen, PhD, Zaven Khachaturian, PhD, Richard Frank, MD, PhD, Peter J. Snyder, PhD, Susan Molchan, PhD, Jeffrey Kaye, MD, Joseph Quinn, MD, Betty Lind, BS, Sara Dolen, BS, Lon S. Schneider, MD, Sonia Pawluczyk, MD, Bryan M. Spann, DO, PhD, James Brewer, MD, PhD, Helen Vanderswag, RN, Judith L. Heidebrink, MD, MS, Joanne L. Lord, LPN, BA, CCRC, Ronald Petersen, MD, PhD, Kris Johnson, RN, Rachelle S. Doody, MD, PhD, Javier Villanueva-Meyer, MD, Munir Chowdhury, MBBS, MS, Yaakov Stern, PhD, Lawrence S. Honig, MD, PhD, Karen L. Bell, MD, John C. Morris, MD, Beau Ances, MD, Maria Carroll, RN, MSN, Sue Leon, RN, MSN, Mark A. Mintun, MD, Stacy Schneider, APRN, BC, GNP, Daniel Marson, JD, PhD, Randall Griffith, PhD, ABPP, David Clark, MD, Hillel Grossman, MD, Effie Mitsis, PhD, Aliza Romirowsky, BA, Leyla deToledo-Morrell, PhD, Raj C. Shah, MD, Ranjan Duara, MD, Daniel Varon, MD, Peggy Roberts, CNA, Marilyn Albert, PhD, Chiadi Onyike, MD, MHS, Stephanie Kielb, MD, Henry Rusinek, PhD, Mony J. de Leon, EdD, Lidia Glodzik, MD, PhD, Susan De Santi, PhD, P. Murali Doraiswamy, MD, Jeffrey R. Petrella, MD, R. Edward Coleman, MD, Steven E. Arnold, MD, Jason H. Karlawish, MD, David Wolk, MD, Charles D. Smith, MD, Greg Jicha, MD, Peter Hardy, PhD, Oscar L. Lopez, MD, MaryAnn Oakley, MA, Donna M. Simpson, CRNP, MPH, Anton P. Porsteinsson, MD, Bonnie S. Goldstein, MS, NP, Kim Martin, RN, Kelly M. Makino, BS, M. Saleem Ismail, MD, Connie Brand, RN, Ruth A. Mulnard, DNSc, RN, FAAN, Gaby Thai, MD, Catherine Mc-Adams-Ortiz, MSN, RN, A/GNP, Kyle Womack, MD, Dana Mathews, MD, PhD, Mary Quiceno, MD, Ramon Diaz-Arrastia, MD, PhD, Richard King, MD, Myron Weiner, MD, Kristen Martin-Cook, MA, Michael DeVous, PhD, Allan I. Levey, MD, PhD, James J. Lah, MD, PhD, Janet S. Cellar, DNP, PMHCNS-BC, Jeffrey M. Burns, MD, Heather S. Anderson, MD, Russell H. Swerdlow, MD, Liana Apostolova, MD, Po H. Lu, PsyD, George Bartzokis, MD, Daniel H.S. Silverman, MD, PhD, Neill R. Graff-Radford, MBBCH, FRCP (London), Francine Parfitt, MSH, CCRC, Heather Johnson, MLS, CCRP, Martin R. Farlow, MD, Ann Marie Hake, MD, Brandy R. Matthews, MD, Scott Herring, RN, Christopher H. van Dyck, MD, Richard E. Carson, PhD, Martha G. MacAvoy, PhD, Howard Chertkow, MD, Howard Bergman, MD, Chris Hosein, MEd, Sandra Black, MD, FRCPC, Bojana Stefanovic, Curtis Caldwell, PhD, Ging-Yuek Robin Hsiung, MD, MHSc, FRCPC, Howard Feldman, MD, FRCPC, Benita Mudge, BS, Michele Assaly, MA, Andrew Kertesz, MD, John Rogers, MD, Dick Trost, PhD, Charles Bernick, MD, Donna Munic, PhD, Diana Kerwin, MD, Marek-Marsel Mesulam, MD, Kristina Lipowski, BA, Chuang-Kuo Wu, MD, PhD, Nancy Johnson, PhD, Carl Sadowsky, MD, Walter Martinez, MD, Teresa Villena, MD, Raymond Scott Turner, MD, PhD, Kathleen Johnson, NP, Brigid Reynolds, NP, Reisa A. Sperling, MD, Keith A. Johnson, MD, Gad Marshall, MD, Meghan Frey, Jerome Yesavage, MD, Joy L. Taylor, PhD, Barton Lane, MD, Allyson Rosen, PhD, Jared Tinklenberg, MD, Marwan Sabbagh, MD, FAAN, CCRI, Christine Belden, PsyD, Sandra Jacobson, MD, Neil Kowall, MD, Ronald Killiany, PhD, Andrew E. Budson, MD, Alexander Norbash, MD, Patricia Lynn Johnson, BA, Thomas O. Obisesan, MD, MPH, Saba Wolday, MSc, Salome K. Bwayo, PharmD, Alan Lerner, MD, Leon Hudson, MPH, Paula Ogrocki, PhD, Evan Fletcher, PhD, Owen Carmichael, PhD, John Olichney, MD, Charles DeCarli, MD, Smita Kittur, MD, Michael Borrie, MB ChB, T.-Y. Lee, PhD, Rob Bartha, PhD, Sterling Johnson, PhD, Sanjay Asthana, MD, Cynthia M. Carlsson, MD, Steven G. Potkin, MD, Adrian Preda, MD, Dana Nguyen, PhD, Pierre Tariot, MD, Adam Fleisher, MD, Stephanie Reeder, BA, Vernice Bates, MD, Horacio Capote, MD, Michelle Rainka, PharmD, CCRP, Douglas W. Scharre, MD, Maria Kataki, MD, PhD, Earl A. Zimmerman, MD, Dzintra Celmins, MD, Alice D. Brown, FNP, Godfrey D. Pearlson, MD, Karen Blank, MD, Karen Anderson, RN, Andrew J. Saykin, PsyD, Robert B. Santulli, MD, Eben S. Schwartz, PhD, Kaycee M. Sink, MD, MAS, Jeff D. Williamson, MD, MHS, Pradeep Garg, PhD, Franklin Watkins, MD, Brian R. Ott, MD, Henry Querfurth, MD, Geoffrey Tremont, PhD, Stephen Salloway, MD, MS, Paul Malloy, PhD, Stephen Correia, PhD, Howard J. Rosen, MD, Bruce L. Miller, MD, Jacobo Mintzer, MD, MBA, Crystal Flynn Longmire, PhD, Kenneth Spicer, MD, PhD, Elizabeth Finger, MD, Irina Rachinsky, MD, John Rogers, MD, Andrew Kertesz, MD and Dick Drost, MD, 7 February 2013, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.1216206110
Other UCLA authors on the paper included Neda Jahanshad, Priya Rajagopalan, Xue Hua, Derrek P. Hibar, Talia M. Nir and Arthur W. Toga.
The project had multiple funding sources, including the National Institutes of Health (grant R01 HD050735). Please see the paper for other authors and additional funding.






Be the first to comment on "New Imaging Method Detects Alzheimer’s Risk Years before the Disease Strikes"