
A study from Scripps Research has made significant advancements in understanding Alzheimer’s disease by employing a novel technique to analyze the electrical activity and protein levels in single neurons. This method, which involves detailed analysis of individual cells, has led to the identification of new molecular targets that could be crucial in developing treatments to slow the disease’s progression. Collaborative efforts by experts in various fields of neuroscience have enabled these breakthroughs, potentially paving the way for new therapeutic strategies.
Researchers have identified new potential drug targets for neurodegenerative diseases by taking detailed electrical and protein measurements from individual brain cells.
The US Centers for Disease Control and Prevention reports that approximately 5.8 million Americans are affected by Alzheimer’s disease, the predominant type of dementia. Currently, there is no cure for Alzheimer’s, largely due to incomplete knowledge about its causes. However, a recent study conducted by Scripps Research is providing new insights into the molecular factors that may influence the progression of Alzheimer’s disease.
In the study, recently published in the journal Advanced Science, the researchers used a new technique for studying single, living brain cells affected by Alzheimer’s disease. By measuring the electrical activity of single neurons and the protein levels within those neurons, the scientists discovered new molecules linked to Alzheimer’s. The hope is these molecules could be targeted by drugs to treat or slow the progression of the neurodegenerative disease in the future.
Close collaboration among Scripps Research’s professors, including clinical neurologist Stuart Lipton, MD, PhD, protein expert John Yates, III, PhD, and bioinformaticist Nicholas Schork, PhD, (who is also the deputy director and distinguished professor of quantitative medicine at The Translational Genomics Research Institute, or TGen) enabled the scientists to develop this biotechnology feat.
The Significance of the Research Technique
“It was mind-boggling to me that we could take one cell, measure its electrical activity on the order of one-millionth of one-millionth of an ampere, and then look at thousands of proteins within that same cell to allow us to find the proteins that drive Alzheimer’s-related abnormal electrical activity,” says senior author Lipton, who is also the Step Family Foundation Endowed Professor and co-director of the Neurodegeneration New Medicines Center at Scripps Research. “But the beauty of this method is that it lets us uncover novel targets for Alzheimer’s disease and related dementias.”
Previous research by Lipton and others has shown that certain neurons become overactive in the brains of people with Alzheimer’s, sending electrical signals that are stronger or more frequent than usual. Evidence suggests that this overactivity (also known as hyperexcitability) contributes to the cognitive decline associated with Alzheimer’s.
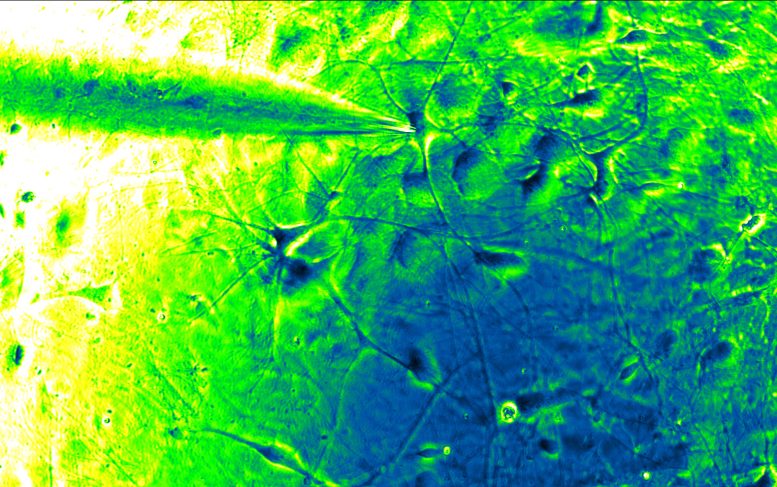
A tiny glass tube (top left) represents the electrode recording from an excitatory human Alzheimer’s neuron, generated using modern stem cell techniques (dark blue, at tip of tube). Credit: Scripps Research
In the new work, Lipton and colleagues developed a system in which scientists can take precise measurements of individual brain cells and then compare those affected by Alzheimer’s with healthy cells. Lipton’s group, who has previously developed methods for precisely measuring the electrical activity of neurons, teamed up with Yates to use mass spectrometry to identify levels of over 2,250 proteins in each nerve cell. Mass spectrometry can identify and quantify proteins from cells, but these analyses have traditionally been done on bulk collections of cells. Recent advances are permitting measurements at the single-cell level.
Advancements in Single-Cell Analysis
In the new system, known as single cell (sc)Patch-Clamp/Proteomics, a tiny glass tube filled with a salt solution is used as an electrode to measure the electrical activity of a cell, and then extract the cell for protein studies with mass spectrometry.
“This approach allows us to connect perturbations of electrical functions to molecular events in neurons, which is an exciting application of proteomics,” says Yates.
The scientists analyzed the electrical patterns and protein levels of about 150 neurons and then used computational tools—applied by Schork—to find associations between hyperexcitability and abnormal protein levels. They pinpointed nearly 50 proteins that were present at higher or lower levels in hyperexcitable Alzheimer’s cells compared to healthy cells.
“Some of these proteins were already known to be associated with Alzheimer’s, but many were not,” says Lipton.
The proteins were involved in many diverse functions of neurons, including control of electrons in free radicals (redox modulators), energy metabolism, and inflammation. Fifteen of the proteins stood out as having particularly high or low levels in Alzheimer’s neurons, and Lipton’s group is planning follow-up studies on some of these molecules.
He also plans to expand the use of scPatch-Clamp/Proteomics for drug screens—testing whether potential Alzheimer’s drugs fix both the hyperexcitability of neurons and the abnormal protein levels. He is correlating these findings with experiments on larger groups of brain cells obtained from patients with Alzheimer’s known as cerebral organoids, or “mini-brains.”
“One cell doesn’t always tell the whole story,” Lipton explains. “Some of the dysfunction in Alzheimer’s has to do with how cells interact with each other, so if we can repeat this kind of study in a mini-brain organoid, we may make additional discoveries.”
Lipton notes this method could be applied to drug discovery efforts for additional brain-related diseases.
“This new approach to personalized medicine—based upon protein expression and electrical activity of a single Alzheimer’s neuron—could revolutionize drug discovery not only for this disease but other neurological diseases, which has lagged far behind other therapeutic areas,” he adds.
Reference: “Single-Cell Patch-Clamp/Proteomics of Human Alzheimer’s Disease iPSC-Derived Excitatory Neurons Versus Isogenic Wild-Type Controls Suggests Novel Causation and Therapeutic Targets” by Swagata Ghatak, Jolene K. Diedrich, Maria Talantova, Nivedita Bhadra, Henry Scott, Meetal Sharma, Matthew Albertolle, Nicholas J. Schork, John R. Yates and Stuart A. Lipton, 21 May 2024, Advanced Science.
DOI: 10.1002/advs.202400545
This work was supported by funding from the National Institutes of Health (R01 AG056259, R35 AG071734, RF1 AG057409, R56 AG065372, R01 DA048882, DP1 DA041722, F32 ES031815, R01MH100175, R21 MH1296776, UH3 AG064706, U19 AG023122, U19 AG065169, and U24 AG078753).






With a family history of dementia and some 5.8 million Americans already affected by Alzheimer’s disease, I can attest to the fact that a treatment to halt and/or reverse the progression of the disease would be a good thing. However, ‘seeing the forest for the cells in the leaves on the trees,’ in the long-run, with a global population of over 8 billion potential victims of dementia of all kinds, prevention would be vastly superior to treatments. However, too, having previously written Dr. Lipton of my own senior lay American male findings on the probable causes of so much now epidemic Alzheimer’s disease (minimally) in the US, what I find “Mind-Boggling” is the failure of so-called “scientists” at Scripps Research to follow-up on what I sent to Dr. Lipton (minimally), about cheaply and easily addressed dietary factors (e.g., https://odysee.com/@charlesgshaver:d?view=about).