
A new quantum sensing tool, Quantum-Enhanced Diamond Molecular Tension Microscopy (QDMTM), developed by teams from the University of Hong Kong and Sichuan University, precisely measures cellular adhesion forces, enhancing our understanding of cell mechanics and aiding cancer research.
Researchers at HKU Engineering develop a breakthrough cellular force imaging technique using a diamond-based quantum sensing microscope.
The project, led by Professor Zhiqin Chu from the Department of Electrical and Electronic Engineering at the University of Hong Kong (HKU), and Professor Qiang Wei from Sichuan University, utilized label-free quantum sensing technology to measure cellular force at the nanoscale. This advancement surpasses the limitations of traditional cellular force measurement tools and provides new insights into cellular mechanics, particularly regarding how cellular adhesion forces affect cancer cell spreading.
The research team has developed a new Quantum-Enhanced Diamond Molecular Tension Microscopy (QDMTM) that offers an effective approach for studying cell adhesion forces. Compared to cell force measurement methods that utilize fluorescent probes, QDMTM has the potential to overcome challenges such as photobleaching, limited sensitivity, and ambiguity in data interpretation. Furthermore, QDMTM sensors can be cleaned and reused, enhancing the absolute accuracy of comparing cell adhesion forces across various samples.
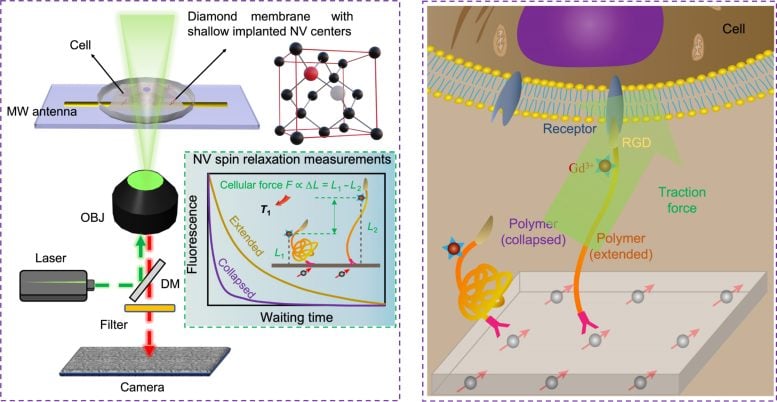
Schematic diagram illustrating the design of QDMTM. The left panel shows the working principle of the widefield quantum diamond microscope. The insert shows how the exerted cellular forces can be quantified by measuring NV centers. The right panel shows the exact force-sensing mechanism. MW antenna: microwave antenna; OBJ: objective; DM: dichroic mirror. Credit: The University of Hong Kong
The new method fundamentally changes the way for studying important issues such as cell-cell or cell-material interactions, with significant implications for biophysics and biomedical engineering. The findings have been published in Science Advances.
Background of the Research
Cells rely on constant interplay and information exchange with their micro-environment to ensure their survival and perform biological functions. Hence, precise quantification of tiny cellular adhesion forces, spanning from piconewtons to a few nanonewtons, is crucial for understanding the intricacies of force modulation in cells.

Professor Zhiqin Chu (right) and Feng Xu (left), the first author of the research paper. Credit: The University of Hong Kong
Over the past few decades, various methods have been successfully developed for measuring cellular adhesive forces. Currently, several leading technologies such as traction force microscopy (TFM), optical/magnetic tweezers, and molecular tension-based fluorescence microscopy (MTFM) are widely used for measuring cellular forces.
However, these techniques have notable limitations in terms of sensitivity and data interpretation, which impede our ability to comprehensively understand mechanobiology. Additionally, the MTFM technique is hindered by the stochastic nature of fluorophore photobleaching. Therefore, it is essential to develop a new technique that can accurately measure cell adhesive forces in a fluorescent label-free manner. This is crucial to advancing the field of mechanobiology.
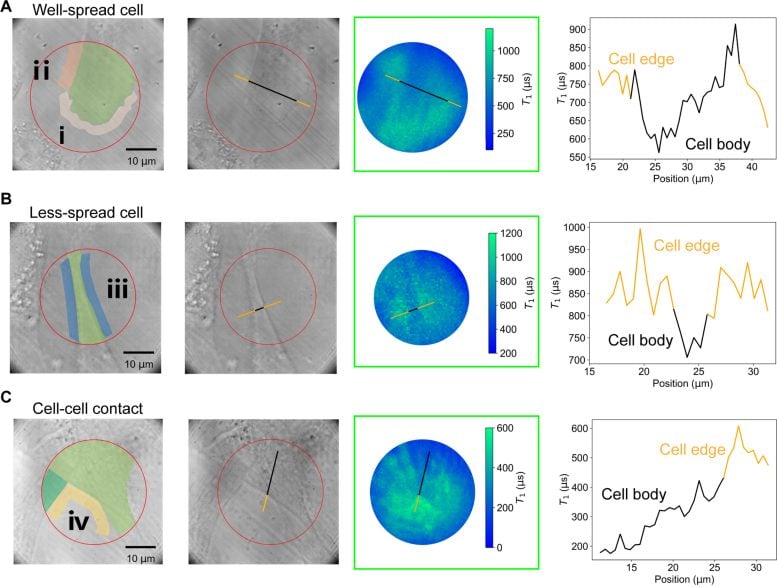
Demonstrated detection of the traction forces of the adhered cells. Three typical cells, namely the (A) well-spread cell, (B) less-spread cell and (C) cell-cell contacted cells were chosen to demonstrate the measurements: the first two columns show the transmitted light images and corresponding marked images; the third column shows the T1 mapping and the last column shows the line profile, as-marked in 2nd and 3rd column, of T1 value across the cell body. (D) T1 values changes in selected areas of cells. Credit: The University of Hong Kong
Research methods and findings
The research team developed QDMTM by combining the extension of polymer (acting as a force transducer) induced by cellular forces with the longitudinal relaxation time of NV. The unique quantum properties of NV center electron spins in diamonds serve as the fundamental basis for the unprecedented sensitivity and precision of QDMTM.
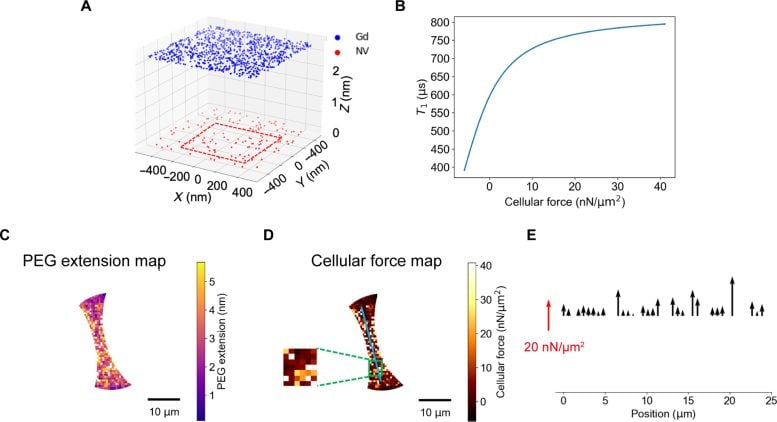
Simulation-assisted extraction of cellular forces from T1 mapping. (A) The schematics show the simplified model: a layer of NV centers is randomly distributed underneath the upper surface of diamond membrane with a given depth and fixed density; a layer of the Gd3+ complexes attached to the PEG acts as a randomly fluctuating spin bath; the aforementioned two layers are separated by spring-like PEG molecules and their force-induced extension is described by Worm-Like Chain model. The red rectangular area represents the minimum sensing area adopted (600 nm × 600 nm). (B) The simulated relationship between cellular forces and T1 value. The extracted (C) PEG extension map, and (D) cellular traction force map of the cell body in one chosen cell (the same one in Fig. 6B). The insert is the enlarged view of the selected rectangular area. (E) Force profile along the blue line drawn in Fig. 7D. The direction of the cellular force exerted on the PEG polymer is normal to the diamond surface. Credit: The University of Hong Kong
The uniqueness of this innovation lies in the utilization of a “force transducer” which is a force-responsive polymer, capable of converting mechanical signals into magnetic signals. By measuring the changes in NV spin relaxation time caused by the magnetic noise, the adhesive forces exerted by cells on the “force transducer” can be determined. Existing measurement techniques are unable to effectively measure the stochastic magnetic signals at the nanoscale.
The innovative QDMTM technique offers an effective approach to studying cell adhesion forces. Through their study, researchers were able to successfully differentiate cells in various adhesion states and found that the magnitude of cellular forces in different cell regions aligned with the previous findings. This suggests that the QDMTM method is capable of accurately measuring cell adhesion forces. The next phase of their research focuses on expanding the quantum sensor from bulk diamond to nanoscale diamond particles, which will allow for the measurement of cell forces in any direction.
Reference: “Quantum-enhanced diamond molecular tension microscopy for quantifying cellular forces” by Feng Xu, Shuxiang Zhang, Linjie Ma, Yong Hou, Jie Li, Andrej Denisenko, Zifu Li, Joachim Spatz, Jörg Wrachtrup, Hai Lei, Yi Cao, Qiang Wei and Zhiqin Chu, 24 January 2024, Science Advances.
DOI: 10.1126/sciadv.adi5300








I make up to $24 an hour working from my home. My story is that I quit working at Walmart to work online and with a little effort I easily bring in around $45h to $89h… Someone was good to me by sharing this link with me, so now I am hoping I could help someone else out there by sharing this link.Try it, you won’t regret it!.
HERE→ https://www.pays77.com