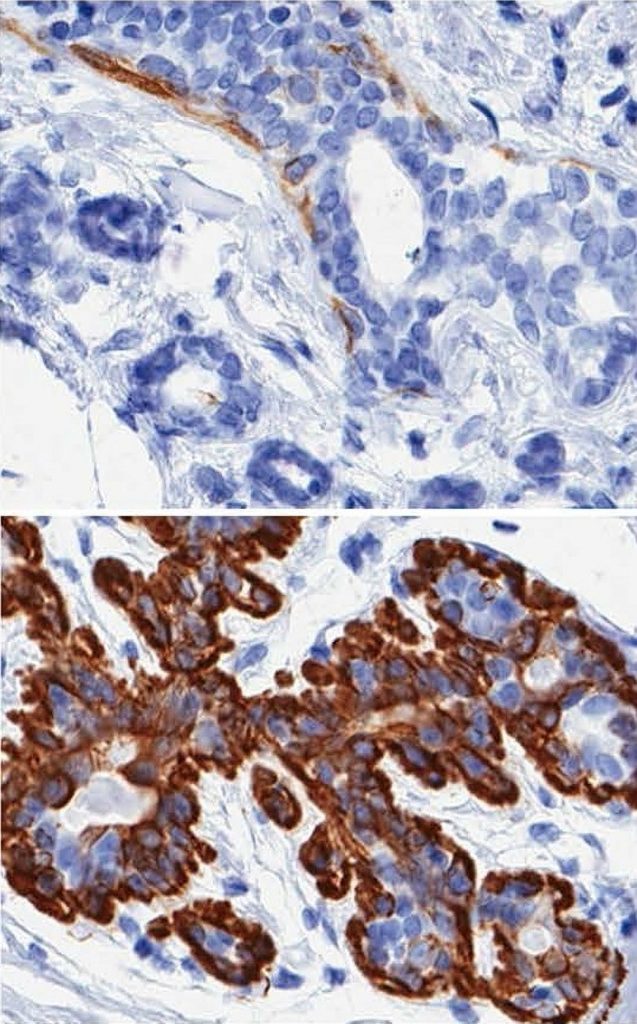
Histological sections of normal breast show lobules from a 37-year-old woman (top) and a 76-year-old woman that were stained to show how the expression of a keratin protein (brown) changes with age. In young women, this protein is only in the cells surrounding the milk-producing cells. In older women even the milk-producing cells make the protein, a factor that may increase the potential for malignant transformation in older women. Credit: Image from Mark LaBarge, Berkeley Lab, and Alexander Barowsky, UC Davis
A new study from the Berkeley Lab takes a step forward in the fight against breast cancer, gaining a better understanding of the cellular basis for age-related vulnerability to breast cancer. During the study, researchers found that that aging causes an increase in multipotent progenitors and a decrease in the myoepithelial cells that line the breast’s milk-producing luminal cells.
It is well-known that the risks of breast cancer increase dramatically for women over the age of 50, but what takes place at the cellular level to cause this increase has been a mystery. Some answers and the possibility of preventative measures in the future are provided in a new study by researchers at the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab).
Mark LaBarge, a cell and molecular biologist in Berkeley Lab’s Life Sciences Division, led a study in which it was determined that aging causes an increase in multipotent progenitors – a type of adult stem cell believed to be at the root of many breast cancers – and a decrease in the myoepithelial cells that line the breast’s milk-producing luminal cells and are believed to serve as tumor suppressors.
“This is a big step towards understanding the cellular basis for age-related vulnerability to breast cancer,” LaBarge says. “Now that we have defined some of the cell and molecular changes that occur in the epithelium during the aging process and we have the ability to assay them functionally, it should be possible to look for ways to avoid those states and perhaps even reverse them.”
LaBarge is the corresponding author of a paper in the journal Cancer Research describing this study. The paper is titled “Accumulation of multipotent progenitors with a basal differentiation bias during aging of human mammary epithelia.” Co-authors are James Garbe, Francois Pepin, Fanny Pelissier, Klara Sputova, Agla Fridriksdottir, Diana Guo, Rene Villadsen, Morag Park, Ole Petersen, Alexander Borowsky and Martha Stampfer.
Each year, more than 200,000 women in the United States are diagnosed with invasive breast cancer and about 75-percent of those women are older than 50. Age-related physiological changes, including endocrine profiles and alterations of the microenvironments surrounding breast cells, have been associated with increased cancer risks, but the underlying cellular mechanisms behind these changes and their links to cancer have not been explained.
“Studying the aging process in any human tissue is a challenge primarily because of limited access to samples,” LaBarge says. “Most studies that have tried get at the cellular or molecular basis of the aging process use model organisms like yeast, flies, worms and mice because their lifespans are short and their genetics are more controlled.”
Human mammary epithelial cells (HMECs) are one of the few examples of an epithelial tissue that affords relatively good access because of mastectomies and cosmetic reduction surgeries. In both cases, surgical discards provide sample tissue for research. In addition, LaBarge and his colleagues also had access to frozen surgical HMEC tissue specimens acquired by co-author Stampfer some 30 years ago when she began developing HMEC cultures for breast cancer research. Using a unique cell culture system developed by Stampfer and co-author Garbe, both of whom are also with Berkeley Lab’s Life Sciences Division, LaBarge and his colleagues were able to generate a large collection of normal HMEC strains derived from primary tissue in women aged 16 to 91 years.

Mark LaBarge, a cell and molecular biologist in Berkeley Lab’s Life Sciences Division, led a research team that took a big step towards understanding the cellular basis for age-related vulnerability to breast cancer. Credit: Photo by Roy Kaltschmidt
“We do not know of another resource in the world like this for studying any type of human tissue,” LaBarge says. “With this resource, we were able to perform functional studies of normal HMECs to observe the cellular changes that occurred with aging.”
He and his colleagues discovered that in finite-lifespan cultured and uncultured epithelial cells, the advancing years usher in a reduction of myoepithelial cells and an increase in luminal cells that express the proteins keratin 14 and integrin α6. In women under 30, these proteins are expressed almost exclusively in myoepithelial cells.
“The aging process therefore results in both a shift in the balance of luminal/myoepithelial lineages and to changes in the functional spectrum of multipotent progenitors that together appear to increase the potential for malignant transformation,” LaBarge says. “We corroborated our culture data with parallel analyses of in vivo samples, but we still have dots to connect to demonstrate that these changes relate to an increased risk of malignancy. All the signs are there, though.”
The collection of normal HMEC strains that the Berkeley research team collected – dubbed the “HMEC Aging Resource” – can be shared with other researchers so that this work can be reproduced or extended.
“Most studies on primary and close-to-primary strains of cells are one-offs, but the cell culture technique we used is so robust that they can be shared,” LaBarge says. “Even pharmaceutical researchers could use these materials for large-scale screenings.”
Reference: “Accumulation of Multipotent Progenitors with a Basal Differentiation Bias during Aging of Human Mammary Epithelia” by James C. Garbe, Francois Pepin, Fanny A. Pelissier, Klara Sputova, Agla J. Fridriksdottir, Diana E. Guo, Rene Villadsen, Morag Park, Ole W. Petersen, Alexander D. Borowsky, Martha R. Stampfer and Mark A. LaBarge, 15 Jult 2012, Cancer Research.
DOI: 10.1158/0008-5472.CAN-12-0157
This research was supported by the DOE Office of Science, Berkeley Lab’s Laboratory Directed Research and Development (LDRD) funding program, and by the National Institute on Aging.








Be the first to comment on "A Step Towards Understanding the Cellular Basis for Age-Related Vulnerability to Breast Cancer"