
New research reveals that managing transposable elements in DNA via the Piwi-piRNA pathway extends lifespan. This finding, which links DNA activity to aging, opens up new possibilities in medical and biological research for health improvement and age determination.
Two Eötvös Loránd University researchers have made an exciting breakthrough in understanding how we age.
Researchers Dr. Ádám Sturm and Dr. Tibor Vellai from Eötvös Loránd University in Hungary have achieved a significant discovery in the study of aging. Their research centered on “transposable elements” (TEs) in our DNA, which are segments capable of relocating within our genetic code. Excessive movement of these TEs can lead to destabilization of the genetic code, potentially contributing to the aging process.
The scientists have identified a specific process, called the Piwi-piRNA pathway, that helps control these TEs. They’ve seen this pathway at work in certain cells that don’t age, like cancer stem cells, and notably, the enigmatic Turritopsis dohrnii, commonly known as the “immortal jellyfish.” By strengthening this pathway in a worm called Caenorhabditis elegans, the worm lived significantly longer.
Groundbreaking Theories and Experimental Proof
In previous landmark articles entitled “The mechanism of aging: primary role of transposable elements in genome disintegration” (2015) and “The Piwi-piRNA pathway: road to immortality” (2017), Dr. Sturm and Dr. Vellai theorized the profound relationship between the Piwi-piRNA system and intriguing concept of biological immortality. Now, in their latest publication in Nature Communications they’ve provided experimental proof. Their research showed that controlling the activity of TEs can indeed extend lifespan, indicating these mobile DNA elements play a crucial role in the aging process.
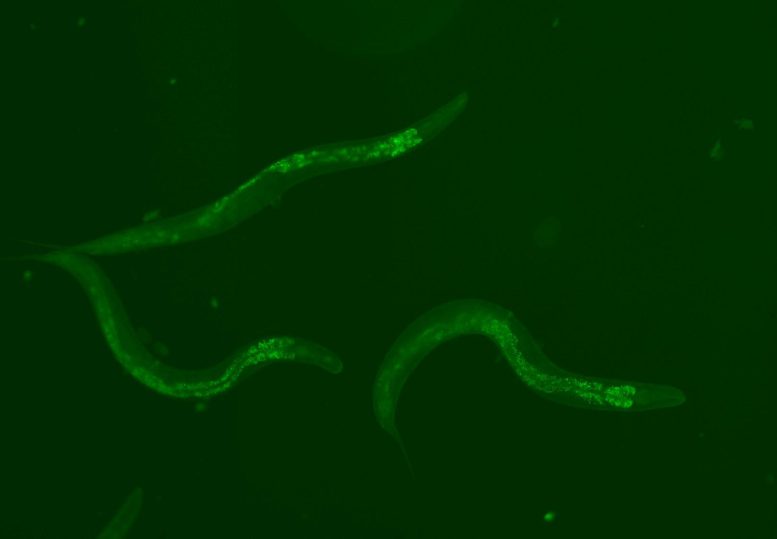
Inside the worms, the reinforced piwi-piRNA pathway lights up green, which enabled them to live longer by 30%. Credit: Sturm, Á., et al., 2023, DBS.
In more technical terms, the researchers used techniques to “downregulate” or quiet down the activity of TEs. When they did this to specific TEs in worms, the worms showed signs of aging slower. Even more, when multiple TEs were controlled simultaneously, the lifespan-extending effects added up.
Implications for Medicine and Biology
“In our lifespan assays, by merely downregulating TEs or somatically overexpressing the Piwi-piRNA pathway elements, we observed a statistically significant lifespan advantage,” Dr. Sturm explained. “This opens the door to a myriad of potential applications in the world of medicine and biology.”
Additionally, the team found epigenetic changes in the DNA of these worms as they aged, specifically in the TEs. These changes, known as DNA N6-adenine methylation, was observed to increase TE transcription and jumping as the animal aged.
Dr. Vellai emphasized the potential implications of this discovery: “This epigenetic modification may pave the way for a method to determine age from DNA, providing an accurate biological clock.”
In conclusion, by better understanding these mobile DNA elements and the pathways that control them, scientists might be on track to developing ways to extend life and improve health in our later years.
Reference: “Downregulation of transposable elements extends lifespan in Caenorhabditis elegans” by Ádám Sturm, Éva Saskői, Bernadette Hotzi, Anna Tarnóci, János Barna, Ferenc Bodnár, Himani Sharma, Tibor Kovács, Eszter Ari, Nóra Weinhardt, Csaba Kerepesi, András Perczel, Zoltán Ivics and Tibor Vellai, 29 August 2023, Nature Communications.
DOI: 10.1038/s41467-023-40957-9








Wonderful website