Researchers have discovered a crucial link between Alzheimer’s disease and fat metabolism, specifically focusing on the amyloid-beta protein and sulfatides. The study, which also considers the impact of diet and lifestyle, opens new possibilities for treatment and prevention. This groundbreaking work could significantly influence future approaches to Alzheimer’s disease.
Recent research by Professors Marcus Grimm and Tobias Hartmann at the Rhineland Campus of SRH University of Applied Health Sciences in Leverkusen and Saarland University has provided new insights into Alzheimer’s disease, potentially paving the way for innovative treatments and prevention methods.
Their study highlights the significant role of bidirectional interactions in the body’s fat metabolism in the development of Alzheimer’s. Additionally, it emphasizes the influence of lifestyle factors like diet and smoking. The team’s findings about the link between the amyloid precursor protein and fat metabolism have been published in the journal Cell Chemical Biology.
Alzheimer’s disease is one of the most common forms of dementia, affecting millions of people worldwide. Patients with Alzheimer’s lose their memory, become disoriented, suffer speech and language impairments, and become increasingly confused as the disease progresses. The disease, in which nerve cells in the brain become damaged and die, is currently incurable.
As the disease develops, countless biochemical processes involving highly complex sequences of commands and signals take place inside the body’s cells. Scientists around the world are currently engaged in research into these complex neural pathways. Understanding what is happening in the body when Alzheimer’s develops offers a chance to intervene and to slow, or ideally stop, the processes involved.
Amyloid-Beta Protein’s Role in Alzheimer’s
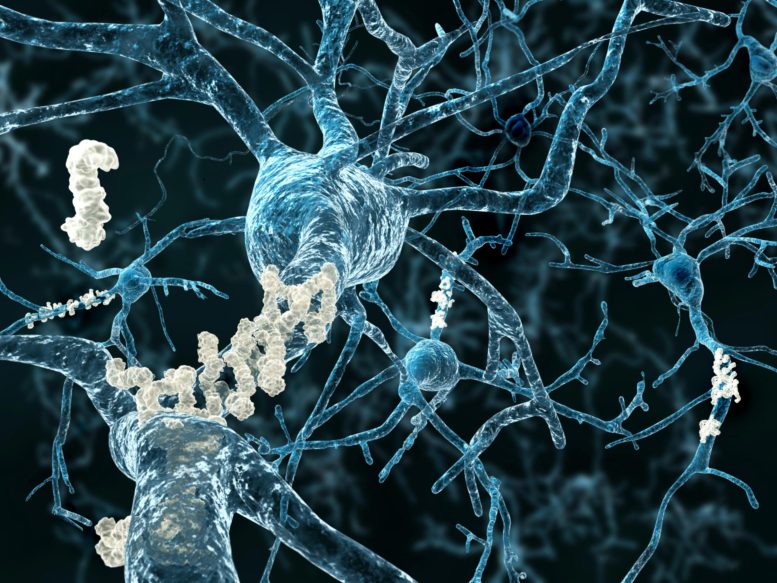
One protein that is known to play a key role in Alzheimer’s disease is the amyloid-beta peptide. In the body of a healthy person, these proteins can be simply broken down. However, in those suffering from Alzheimer’s, they clump together to form ‘plaques’ that are deposited between the nerve cells of the brain.
‘This small amyloid-beta protein accumulates in the form of hardened plaques within a patient’s brain. Amyloid-beta is a key element in the development of Alzheimer’s and leads to neurodegeneration,’ explains nutrition specialist Professor Marcus Grimm, who teaches and researches at the Rhineland Campus of the SRH University of Applied Health Sciences in Leverkusen and at Saarland University, where he collaborates closely with Professor Tobias Hartmann, who heads the German Institute for Dementia Prevention on the university’s medical campus in Homburg, Saarland. Marcus Grimm heads a molecular and cell biology research lab at the Homburg institute.
The Connection Between Alzheimer’s and Diet
Professors Hartmann and Grimm have long been on the trail of how Alzheimer’s and diet are connected, and their research team has now found new evidence supporting just such a link. The team has succeeded in identifying a previously unknown mechanism in the body’s fat metabolism that can lead to the development of Alzheimer’s.
They discovered that the production of the amyloid-beta protein influences the synthesis of certain fats, particularly a class of lipids known as sulfatides, and, conversely, that the quantity of sulfatides mediates the amount of amyloid-beta. This bidirectional interaction is of potentially major significance in Alzheimer’s research, as the level of sulfatides is known to be depleted and the level of amyloid-beta elevated in the brains of Alzheimer’s patients.
The Study’s Findings and Implications
‘Our study has identified a previously unknown physiological aspect of how the amyloid precursor protein (APP) is processed, and this is significant because APP plays a key role in regulating the metabolism of lipids, particularly sulfatides, in the brain. Sulfatides are special fats that are present in the food we eat but that can also be produced by the body itself,’ explained Marcus Grimm, who at SRH University of Applied Health Sciences also heads the Bachelor’s degree program in Nutrition Therapy and Nutrition Counselling and the Master’s degree program in Medical Nutrition Science and Nutrition Therapy.
‘We have been able to demonstrate experimentally that amyloid-beta production influences the amount of sulfatides and vice versa. Our results show that the cleavage of the precursor protein to produce amyloid-beta also leads to the release of another protein fragment called AICD. AICD in turn inhibits expression of the enzyme Gal3st1/CST, which plays a central role in the body’s own sulfatide synthesis,’ said Grimm, explaining the complex metabolic processes that occur in the cells of Alzheimer’s patients.
Of particular interest to the researchers is the impact that diet and lifestyle may have on the disease. ‘Factors such as smoking can have a negative effect on sulfatide levels, whereas ensuring the body has an adequate supply of vitamin K or eating certain types of seafood can have a positive effect. These findings suggest possible approaches to developing preventive and therapeutic strategies in the fight against Alzheimer’s disease,’ said Professor of Experimental Neurology Tobias Hartmann.
‘Our study emphasizes the importance of an intact biochemical circuit that regulates sulfatide homeostasis and amyloid-beta production and shows that this regulatory circuit is disrupted in Alzheimer’s patients.’ The new insights that this research offers into the physiological processes that accompany the development of Alzheimer’s may open up new avenues in the treatment of the disease.
Reference: “A bidirectional link between sulfatide and Alzheimer’s disease” by Valerie Christin Zimmer, Anna Andrea Lauer, Viola Haupenthal, Christoph Peter Stahlmann, Janine Mett, Sven Grösgen, Benjamin Hundsdörfer, Tatjana Rothhaar, Kristina Endres, Matthias Eckhardt, Tobias Hartmann, Heike Sabine Grimm and Marcus Otto Walter Grimm, 15 November 2023, Cell Chemical Biology.
DOI: 10.1016/j.chembiol.2023.10.021








Be the first to comment on "Alzheimer’s Breakthrough: Fat Metabolism Key to New Treatments"