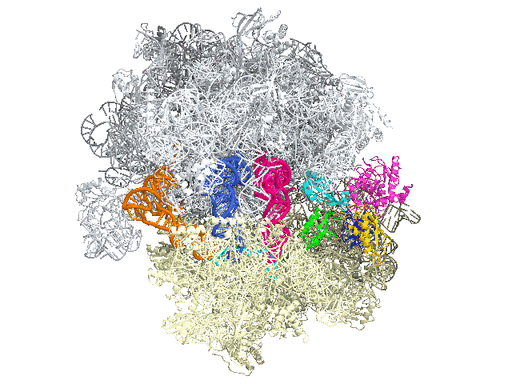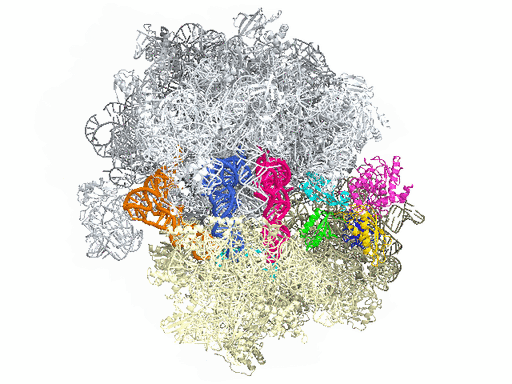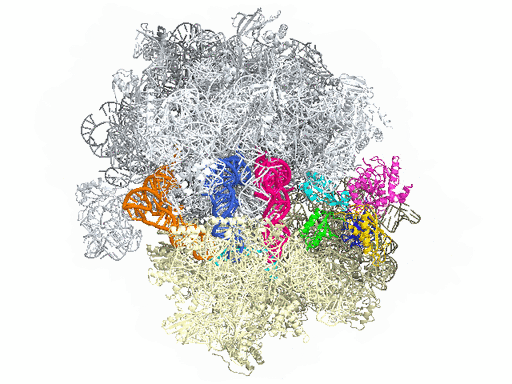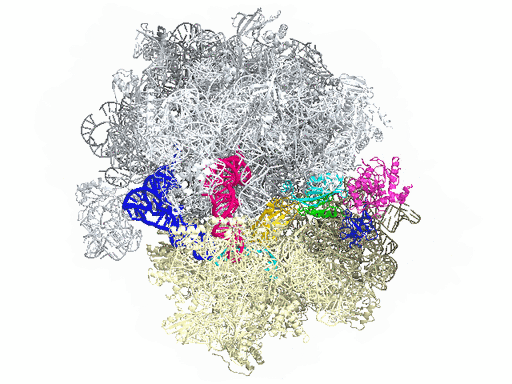
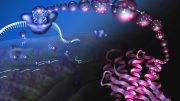
The ribosome acts as a protein-making “factory” in cells, assembling amino acids into polypeptide chains that constitute proteins. Credit: Yale University
New research from Yale University provides insights into the conformational space that EF-G samples on the ribosome and reveals that tRNA translocation on the ribosome is facilitated by a structural transition of EF-G from a compact to an elongated conformation, which can be prevented by the antibiotic dityromycin.
The ribosome is the protein-making “factory” within cells responsible for knitting together amino acids into polypeptide chains that form proteins.
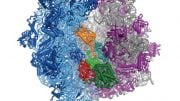
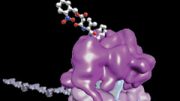
This process requires numerous factors. One of the most crucial is elongation factor G (EF-G), shown in color in the accompanying illustration of the ribosome. EF-G is responsible for movement within the ribosome of messenger RNA (mRNA) and transfer RNA (tRNA), the molecules that carry out instructions contained in DNA. But how does this happen?
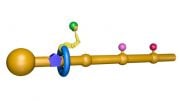
In this simplified illustration, Yale research scientist Jinzhong Lin and colleagues in the lab of Nobel laureate Thomas Steitz in the Department of Molecular Biophysics and Biochemistry show that portions of EF-G (in green, yellow, and red) function something like a motor and force tRNA to move forward after the addition of each amino acid, a necessary step to add to the developing polypeptide chain.
Reference: “Conformational Changes of Elongation Factor G on the Ribosome during tRNA Translocation” by Jinzhong Lin, Matthieu G. Gagnon, David Bulkley and Thomas A. Steitz, 15 January 2015, Cell.
DOI: 10.1016/j.cell.2014.11.049





Be the first to comment on "Conformational Changes of EF-G on the Ribosome During tRNA Translocation"