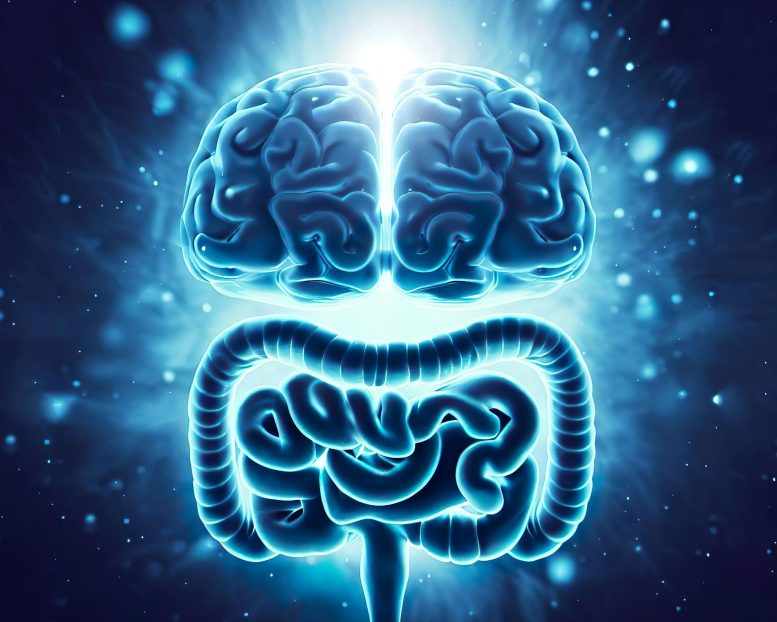
The enteric nervous system (ENS), often called the second brain, plays a crucial role in digestion, immunity, and communication with the brain. Researchers have discovered that ENS development continues after birth and includes neurons derived from mesoderm, challenging long-held scientific beliefs and opening avenues for potential new treatments for aging and gastrointestinal diseases.
Discoveries may pave the way for improved therapies for gastrointestinal issues.
Following your gut. Losing your appetite. A gutsy move. Though we often consider the gut as merely a digestive tool, these common expressions reflect the central role the gut plays in a much wider range of essential functions.
The entire digestive tract is lined by the enteric nervous system (ENS), a vast network of millions of neurons and glial cells—the two primary cell types also found in the central nervous system. While often called the second brain, the ENS not only generates the same neurotransmitters but actually predates the evolution of the central nervous system in the brain.
The functions of the ENS are crucial to life and extend far beyond digestion, as it regulates immunity, gut secretions, and enables complex, bi-directional communication between the gut and the brain. This is why a happy gut co-exists with a happy brain, and why digestive issues can lead to changes in mood and behavior.
Since the mid-20th century, scientists have believed that the ENS is derived from the neural crest before birth and remains unchanged after. Now, in a paper published in the journal eLife, researchers at Beth Israel Deaconess Medical Center (BIDMC) present a completely new paradigm describing a developmental pathway by which ENS development continues after birth in mice and human tissue samples.
This discovery overturns decades of scientific dogma on the fundamental biology of neuroscience and of ENS, by showing evidence for the first time of a non-ectodermal and a mesodermal origin for large numbers of enteric neurons born after birth. The findings show the relevance of these neurons to the maturation and aging of the ENS in health and disease.
“These results indicate for the first time that the mesoderm is an important source of neurons in the second largest nervous system of the body,” said Subhash Kulkarni, Ph.D., a staff scientist at BIDMC and an assistant professor in the Division of Medical Sciences at Harvard Medical School. “How we mature and how we age is central to our understanding of health and disease in our rapidly aging population. The increasing proportion of neurons of mesodermal lineage is a natural consequence of maturation and aging; further, this lineage can be expected to have distinct vulnerabilities to disease.”
Using transgenic mice models, high-resolution microscopy, and genetic analyses, Kulkarni and colleagues analyzed the ENS neuronal populations in adult mice and human tissues. Using mice models, the team found that while the early post-natal ENS cells were from the expected neural crest lineage, that pattern changed rapidly as the animal matured. Kulkarni and colleagues documented the arrival and continual expansion of a novel population of enteric neurons that were derived from the mesoderm—the same lineage that gives rise to the muscle and heart cells.
This newly discovered population of mesoderm-derived neurons expanded with age, such that they comprised a third of all enteric neurons in adolescent mice, half of all enteric neurons in adult mice, and then eventually outnumbered the original neural crest-derived population of enteric neurons in aging mice.
By assessing the molecular signature of these neurons, the team identified new cellular markers that were used to identify this population of mesoderm-derived neurons in human gut tissue. These markers also provided pharmacological targets, which the researchers used to not only manipulate the proportions of the mesodermal neurons in adolescent mice but also reduce their dominant proportions in the aging mouse gut to cure age-associated slowing of gut movement.
“We can now work to understand how these findings can be translated into human systems to provide a disease-modifying cure to aging patients whose chief complaint often includes diseases of the GI tract,” added Kulkarni. “By reversing one of the biggest dogmas of neuroscience, we are now in uncharted territory and, at the same time, have a huge opportunity to understand this hidden basic, translational, and clinical biology of neurons. The newly discovered lineage of neurons presents us with potential new drug targets that could help large populations of patients.”
Reference: “Age-associated changes in lineage composition of the enteric nervous system regulate gut health and disease” by Subhash Kulkarni, Monalee Saha, Jared Slosberg, Alpana Singh, Sushma Nagaraj, Laren Becker, Chengxiu Zhang, Alicia Bukowski, Zhuolun Wang, Guosheng Liu, Jenna Leser, Mithra Kumar, Shriya Bakhshi, Matthew Anderson, Mark Lewandoski, Elizabeth Vincent, Loyal A. Goff and Pankaj Jay Pasricha, 7 August 2023, eLife.
DOI: 10.7554/eLife.88051.1
Co-authors included Monalee Saha, Jared Slosberg, Alpana Singh, Sushma Nagaraj, Chengxiu Zhang, Alicia Bukowski, Zhuolun Wang, Guosheng Liu, Jenna Leser, Mithra Kumar, Shriya Bakhshi, Elizabeth Vincent, and Loyal A. Goff of Johns Hopkins University School of Medicine; Laren Becker and of Stanford University School of Medicine; Matthew Anderson and Mark Lewandoski of Center for Cancer Research, National Cancer Institute; and Pankaj Jay Pasricha of the Mayo Clinic.
The microscopy was performed on the Ross Imaging Core at the Hopkins Conte Digestive Disease Center at the Johns Hopkins University (P30DK089502) using the Olympus FV 3000rs (procured with the NIH-NIDDK S10 OD025244 grant). The 10X Genomics Chromium processing for scRNAseq was performed at the GRCF Core and the sequencing was performed at the CIDR core at the Johns Hopkins University. This work was supported through a grant from the Ludwig Foundation, a grant from the NIA (R01AG066768), a pilot award from the Hopkins Digestive Diseases Basic & Translational Research Core Center grant (P30DK089502), a pilot award from the Diacomp initiative through Augusta University; a Johns Hopkins Catalyst Award; the Maryland Genetics, Epidemiology, and Medicine training program sponsored by the Burroughs Welcome Fund; the Hopkins Conte Digestive Disease Center at the Johns Hopkins University (P30DK089502); NIDDK (R01DK080920); the Maryland Stem Cell Research Foundation (MSCRF130005), and a grant from the AMOS family.







I have believed for many years that the “second brain” ….. is, in reality, the first brain. Based on the primacy of the GI tract in developmental terms.
Doubtless the gut brain will be found to be the source for gender dysphoria, thus placing transgenderism on solid scientific footing.
lol
FJB
Total BS…glyphosate used to protect nutrients deleted from GM/GMO food crops and needed by the intestinal Mucosal immune system to function properly are combined to produce dozens of chronic diseases.
The article describing new developments in the understanding and expanding our knowledge of the Gut Brain is illuminating. The article from a layperson ‘s reading was readable and satisfying.