Nasal vaccines are a type of immunization that are administered through the nasal passages rather than through injections. The advantage of nasal vaccines is that they offer a less invasive alternative to traditional injections, and they have been shown to be effective in providing immunity. Additionally, because they do not require the use of needles, they may be a good option for people who are needle-phobic or for children who may be afraid of shots.
Since the outbreak of COVID-19, scientists have been exploring the development of mucosal vaccines that can be delivered through the nasal route. Recently, researchers in Berlin have successfully created a live attenuated vaccine for the nose. In a recent study, they detail the unique immune response it elicits.
The spread of coronaviruses primarily occurs through the air when infected individuals expel droplets of saliva containing the virus through speaking, coughing, sneezing, or laughing. These airborne pathogens are then inhaled by others, leading to infection. In an effort to combat the virus that causes COVID-19, a research team in Berlin has decided to target the initial point of entry, the mucous membranes of the nose, mouth, throat, and lungs.
To do so, the scientists developed a live attenuated SARS-CoV-2 vaccine that is administered through the nose. In the latest issue of the journal Nature Microbiology, the interdisciplinary team describes how this live attenuated vaccine confers better immunity than vaccines injected into muscle.
Already in the fall of last year, two nasal vaccination formulations were approved for use in India and China. These contain modified adenoviruses – which typically cause respiratory or gastrointestinal illnesses – that are self-attenuating, meaning they either replicate poorly or stop replicating altogether, and therefore never trigger disease. Other live nasal vaccines are currently undergoing development and testing around the world.
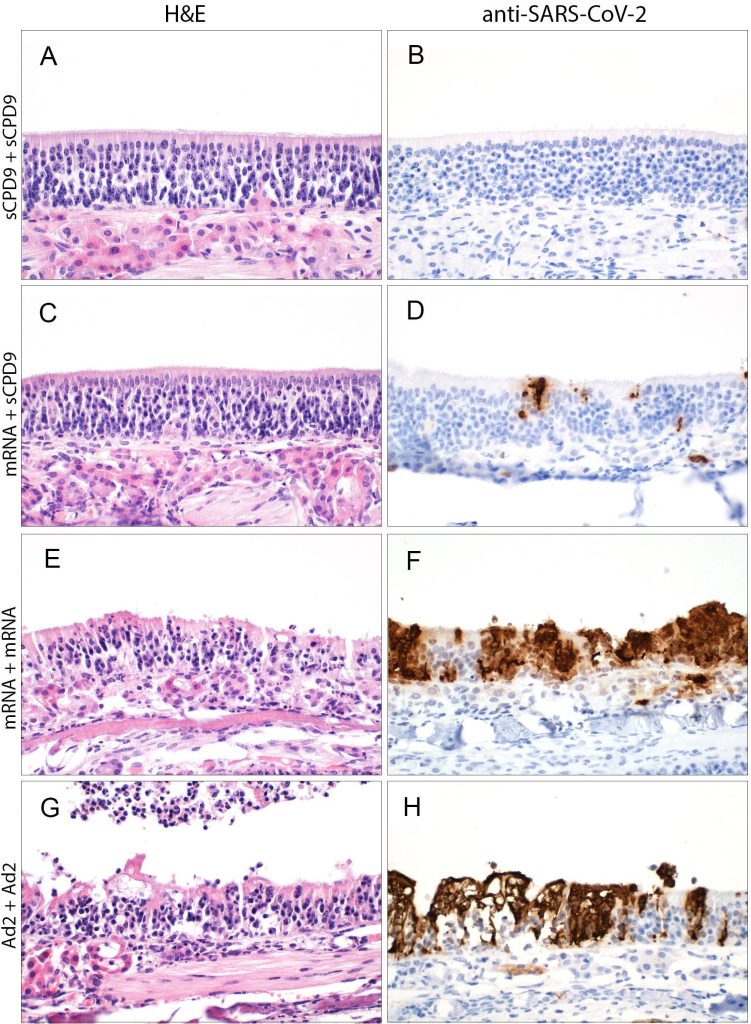
After double vaccination with the live attenuated vaccine (A), the nasal mucosa in the hamster model is very well protected and shows hardly any changes from SARS-CoV-2 (B). The combination of live and mRNA vaccines (C) is also very effective, but the virus still finds small sites to attack (stained brown) in the nasal mucosa (D). In comparison, double intramuscular vaccines perform much worse in terms of protecting the nasal mucosa (E+F and G+H). They allow the virus to damage the upper tissue layers. Credit: Anne Voß, Institute of Veterinary Pathology, Freie Universität Berlin
Protection at the site of infection
The benefits of a nasal vaccine go far beyond just providing an alternative for people afraid of needles. When a vaccine is injected, it infers immunity primarily in the blood and throughout the entire body. However, this means that the immune system only detects and combats coronaviruses relatively late on in an infection, as they enter the body via the mucous membranes of the upper respiratory tract. “It is here, therefore, that we need local immunity if we want to intercept a respiratory virus early on,” explains the study’s co-last author Dr. Jakob Trimpert, a veterinarian and research group leader at the Institute of Virology at Freie Universität Berlin.
“Nasal vaccines are far more effective in this regard than injected vaccines, which fail or struggle to reach the mucous membranes,” emphasizes Dr. Emanuel Wyler, another co-last author. He has been researching COVID-19 since the start of the pandemic as part of the RNA Biology and Posttranscriptional Regulation Lab, which is led by Professor Markus Landthaler at the Berlin Institute for Medical Systems Biology of the Max Delbrück Center (MDC-BIMSB).
In an ideal scenario, a live intranasal vaccine stimulates the formation of the antibody immunoglobulin A (IgA) directly on site, thus preventing infection from occurring in the first place. IgA is the most common immunoglobin in the mucous membranes of the airways. It is able to neutralize pathogens by binding to them and preventing them from infecting respiratory tract cells. At the same time, the vaccine stimulates systemic immune responses that help provide effective overall protection from infection.
“Memory T cells that reside in lung tissue play a similarly useful role to antibodies in the mucosa,” explains Dr. Geraldine Nouailles, an immunologist and research group leader at the Department of Pneumology, Respiratory Medicine, and Intensive Care Medicine at Charité. “These white blood cells remain in affected tissue long after an infection has passed and remember pathogens they have encountered before. Thanks to their location in the lungs, they can respond quickly to viruses that enter through the airways.” The co-first author draws attention to one of the observations the team made during their study: “We were able to show that prior intranasal vaccination results in the increased reactivation of these local memory cells in the event of a subsequent SARS-CoV-2 infection. Needless to say, we were particularly pleased with this result.”
Local immunity impedes viral infection
The scientists tested the efficacy of the newly developed intranasal COVID-19 vaccine on hamster models that had been established by Trimpert and his team at Freie Universität Berlin at the beginning of the pandemic. These rodents are currently the most important non-transgenic model organisms for research into the novel coronavirus, as they can be infected with the same virus variants as humans and develop similar symptoms. They found that after two doses of the vaccine, the virus could no longer replicate in the model organism. “We witnessed strong activation of the immunological memory, and the mucous membranes were very well protected by the high concentration of antibodies,” Trimpert explains. The vaccine could therefore also significantly reduce the transmissibility of the virus.
In addition, the scientists compared the efficacy of the live attenuated vaccine with that of vaccines injected into the muscle. To do so, they vaccinated the hamsters either twice with the live vaccine, once with the mRNA and once with the live vaccine, or twice with an mRNA or adenovirus-based vaccine. Then, after the hamsters were infected with SARS-CoV-2, they used tissue samples from the nasal mucosa and lungs to see how strongly the virus was still able to attack the mucosal cells. They also determined the extent of the inflammatory response using single-cell sequencing. “The live attenuated vaccine performed better than the other vaccines in all parameters,” Wyler summarizes. This is probably due to the fact that the nasally administered vaccine builds up immunity directly at the viral entry site. In addition, the live vaccine contains all components of the virus – not just the spike protein, as is the case with the mRNA vaccines. While spike is indeed the virus’s most important antigen, the immune system can also recognize the virus from about 20 other proteins.
Better than conventional vaccines
The best protection against the SARS-CoV-2 was provided by double nasal vaccination, followed by the combination of a muscular injection of the mRNA vaccine and the subsequent nasal administration of the live attenuated vaccine. “This means the live vaccine could be particularly interesting as a booster,” says the study’s co-first author Julia Adler, a veterinarian and doctoral student at the Institute of Virology at Freie Universität Berlin.
The principle of live attenuated vaccines is old and is already used in measles and rubella vaccinations, for example. But in the past, scientists generated the attenuation by chance – sometimes waiting years for mutations to evolve that produced an attenuated virus. The Berlin researchers, on the other hand, were able to specifically alter the genetic code of the coronaviruses. “We wanted to prevent the attenuated viruses from mutating back into a more aggressive variant,” explains Dr. Dusan Kunec, a scientist at the Institute of Virology at Freie Universität Berlin and another co-last author of the study. “This makes our live vaccine entirely safe and means it can be tailored to new virus variants,” stresses Kunec, who was instrumental in developing the vaccine.
The next step is safety testing: The researchers are collaborating with RocketVax AG, a Swiss start-up based in Basel. The biotech company is developing the live attenuated SARS-CoV-2 vaccine and preparing a phase 1 clinical trial in humans. “We are thrilled to be at the forefront of developing and manufacturing the live attenuated SARS-CoV-2 vaccine as a nasal spray at RocketVax. Our goal is to rapidly scale up production and advance clinical development towards market access to provide protection against post-COVID symptoms for all. We see great potential in the market for seasonal nasal vaccines”, says Dr. Vladimir Cmiljanovic, CEO of RocketVax.
The future will show which nasal vaccine will ultimately provide better protection. The manufacturers of the nasal adenovirus vaccines developed in India and China have not yet applied for approval in Europe. But one thing is clear to the scientists: since they are administered as nasal sprays or drops, nasal vaccines are a good option for use in places with limited access to trained medical staff. They are also inexpensive to produce and easy to store and transport. Last but not least, live attenuated vaccines such as this one have been proven to provide cross-protection against related viral strains, and thus presumably also against future SARS-CoV-2 variants.
Reference: “Live-attenuated vaccine sCPD9 elicits superior mucosal and systemic immunity to SARS-CoV-2 variants in hamsters” by Geraldine Nouailles, Julia M. Adler, Peter Pennitz, Stefan Peidli, Luiz Gustavo Teixeira Alves, Morris Baumgardt, Judith Bushe, Anne Voss, Alina Langenhagen, Christine Langner, Ricardo Martin Vidal, Fabian Pott, Julia Kazmierski, Aileen Ebenig, Mona V. Lange, Michael D. Mühlebach, Cengiz Goekeri, Szandor Simmons, Na Xing, Azza Abdelgawad, Susanne Herwig, Günter Cichon, Daniela Niemeyer, Christian Drosten, Christine Goffinet, Markus Landthaler, Nils Blüthgen, Haibo Wu, Martin Witzenrath, Achim D. Gruber, Samantha D. Praktiknjo, Nikolaus Osterrieder, Emanuel Wyler, Dusan Kunec, and Jakob Trimpert, 3 April 2023, Nature Microbiology.
DOI: 10.1038/s41564-023-01352-8
The study was funded by the German Research Foundation (DFG). The Berlin researchers are collaborating with the Swiss company RocketVax AG on the further development of the vaccine.





Be the first to comment on "Game-Changing COVID-19 Nasal Vaccine Passes Initial Tests"