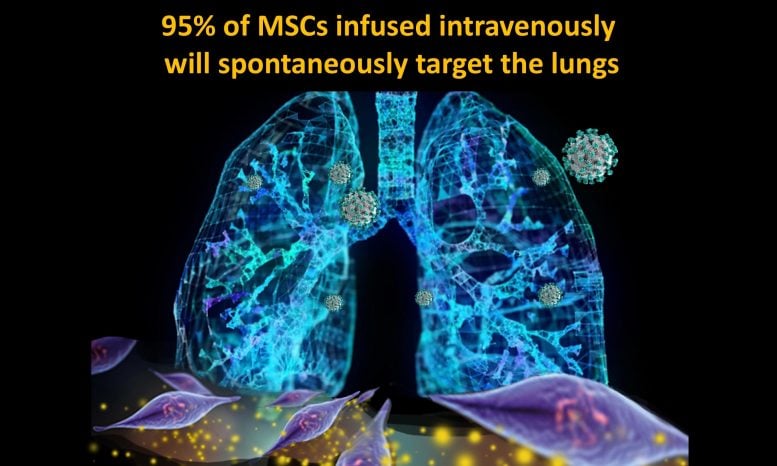
Umbilical cord-derived mesenchymal stem cells naturally migrate directly to the lung where they begin repair to COVID-19 damage. Credit: © Dr. Camillo Ricordi
Study looked at treating severe COVID-19 with umbilical-cord derived mesenchymal stem cells.
University of Miami Miller School of Medicine researchers led a unique and groundbreaking randomized controlled trial showing umbilical cord derived mesenchymal stem cell infusions safely reduce risk of death and quicken time to recovery for the severest COVID-19 patients, according to results published in STEM CELLS Translational Medicine in January 2021.
The study’s senior author, Camillo Ricordi, M.D., director of the Diabetes Research Institute (DRI) and Cell Transplant Center at the University of Miami Miller School of Medicine, said treating COVID-19 with mesenchymal stem cells makes sense.
Results: treatment group vs. control group
The paper describes findings from 24 patients hospitalized at University of Miami Tower or Jackson Memorial Hospital with COVID-19 who developed severe acute respiratory distress syndrome. Each received two infusions given days apart of either mesenchymal stem cells or placebo.
“It was a double-blind study. Doctors and patients didn’t know what was infused,” Dr. Ricordi said. “Two infusions of 100 million stem cells were delivered within three days, for a total of 200 million cells in each subject in the treatment group.”
Researchers found the treatment was safe, with no infusion-related serious adverse events.

Camillo Ricordi, M.D., director of the Diabetes Research Institute (DRI) and Cell Transplant Center at the University of Miami Miller School of Medicine. Credit: University of Miami Health System
Patient survival at one month was 91% in the stem cell treated group versus 42% in the control group. Among patients younger than 85 years old, 100% of those treated with mesenchymal stem cells survived at one month.
Dr. Ricordi and colleagues also found time to recovery was faster among those in the treatment arm. More than half of patients treated with mesenchymal stem cell infusions recovered and went home from the hospital within two weeks after the last treatment. More than 80% of the treatment group recovered by day 30, versus less than 37% in the control group.
“The umbilical cord contains progenitor stem cells, or mesenchymal stem cells, that can be expanded and provide therapeutic doses for over 10,000 patients from a single umbilical cord. It’s a unique resource of cells that are under investigation for their possible use in cell therapy applications, anytime you have to modulate immune response or inflammatory response,” he said. “We’ve been studying them with our collaborators in China for more than 10 years in Type 1 Diabetes, and there are currently over 260 clinical studies listed in clinicaltrials.gov for treatment of other autoimmune diseases.”
Mesenchymal stem cells potential to restore normal immune response
Mesenchymal cells not only help correct immune and inflammatory responses that go awry, they also have antimicrobial activity and have been shown to promote tissue regeneration.
“Our results confirm the powerful anti-inflammatory, immunomodulatory effect of UC-MSC. These cells have clearly inhibited the ‘cytokine storm’, a hallmark of severe COVID-19,” said Giacomo Lanzoni, Ph.D, lead author of the paper and assistant research professor at the Diabetes Research Institute. “The results are critically important not only for COVID-19 but also for other diseases characterized by aberrant and hyperinflammatory immune responses, such as autoimmune Type 1 Diabetes.”
When given intravenously, mesenchymal stem cells migrate naturally to the lungs. That’s where therapy is needed in COVID-19 patients with acute respiratory distress syndrome, a dangerous complication associated with severe inflammation and fluid buildup in the lungs.
“It seemed to me that these stem cells could be an ideal treatment option for severe COVID-19,” said Dr. Ricordi, Stacy Joy Goodman Professor of Surgery, Distinguished Professor of Medicine, and professor of biomedical engineering, microbiology and immunology. “It requires only an intravenous (IV) infusion, like a blood transfusion. It’s like smart bomb technology in the lung to restore normal immune response and reverse life-threatening complications.”
Early success with mesenchymal stem cells
When the pandemic emerged, Dr. Ricordi asked collaborators in China if they had studied mesenchymal stem cell treatment in COVID-19 patients. In fact, they and Israeli researchers reported great success treating COVID-19 patients with the stem cells, in many cases with 100% of treated patients surviving and recovering faster than those without stem cell treatment.
But there was widespread skepticism about these initial results, because none of the studies had been randomized, where patients randomly received treatment or a control solution (placebo), to compare results in similar groups of patients.
“We approached the FDA and they approved our proposed randomized controlled trial in one week, and we started as quickly as possible,” Dr. Ricordi said.
Dr. Ricordi worked with several key collaborators at the Miller School, the University of Miami Health System, Jackson Health System, and collaborated with others in the U.S. and internationally, including Arnold I. Caplan, Ph.D., of Case Western Reserve University, who first described mesenchymal stem cells.
Next steps
The next step is to study use of the stem cells in COVID-19 patients who have not yet become severely ill but are at risk of having to be intubated, to determine if the infusions prevent disease progression.
The findings have implications for studies in other diseases, too, according to Dr. Ricordi.
Hyper-immune and hyper-inflammatory responses in autoimmune diseases might share a common thread with why some COVID-19 patients transition to severe forms of the disease and others don’t.
“Autoimmunity is a big challenge for healthcare, as is COVID-19. Autoimmunity affects 20% of the American population and includes over 100 disease conditions, of which Type 1 Diabetes can be considered just the tip of the iceberg. What we are learning is that there may be a common thread and risk factors that can predispose to both an autoimmune disease or to a severe reaction following viral infections, such as SARS-CoV-2,” he said.
The DRI Cell Transplant Center is planning to create a large repository of mesenchymal stem cells that are ready to use and can be distributed to hospitals and centers in North America, he said.
“These could be used not only for COVID-19 but also for clinical trials to treat autoimmune diseases, like Type 1 Diabetes,” Dr. Ricordi said. “If we could infuse these cells at the onset of Type 1 Diabetes, we might be able to block progression of autoimmunity in newly diagnosed subjects, and progression of complications in patients affected by the disease long-term. We are planning such a trial specifically for diabetes nephropathy, a kidney disease that is one of the major causes of dialysis and kidney transplantation. We are also planning to do a study on umbilical cord mesenchymal stem cell transplantation in combination with pancreatic islets to see if you can modulate the immune response to an islet transplant locally.”
Funding by The Cure Alliance made launching the initial trial possible, while a $3 million grant from North America’s Building Trades Unions (NABTU) allowed Dr. Ricordi and colleagues to complete the clinical trial and expand research with mesenchymal stem cells.
“North America’s Building Trades Unions (NABTU) has been a major supporter of the Diabetes Research Institute since 1984, when they started a campaign to fund, and build, our state-of-the-art research and treatment facility. NABTU has continued to support our work through the years, including our mesenchymal stem cell research that helped lead the way to this clinical trial,” he said.
Reference: “Umbilical cord mesenchymal stem cells for COVID‐19 acute respiratory distress syndrome: A double‐blind, phase 1/2a, randomized controlled trial” by Giacomo Lanzoni, Elina Linetsky, Diego Correa, Shari Messinger Cayetano, Roger A. Alvarez, Dimitrios Kouroupis, Ana Alvarez Gil, Raffaella Poggioli, Phillip Ruiz, Antonio C. Marttos, Khemraj Hirani, Crystal A. Bell, Halina Kusack, Lisa Rafkin, David Baidal, Andrew Pastewski, Kunal Gawri, Clarissa Leñero, Alejandro M. A. Mantero, Sarah W. Metalonis, Xiaojing Wang, Luis Roque, Burlett Masters, Norma S. Kenyon, Enrique Ginzburg, Xiumin Xu, Jianming Tan, Arnold I. Caplan, Marilyn K. Glassberg, Rodolfo Alejandro and Camillo Ricordi, 5 January 2021, STEM CELLS Translational Medicine.
DOI: 10.1002/sctm.20-0472
All the organizations funding the research are nonprofit entities, including the Barilla Group and Family, The Fondazione Silvio Tronchetti Provera, the Simkins Family Foundation and the Diabetes Research Institute Foundation. The National Center for Advancing Translational Sciences also provided funding.
Coauthors on the NEJM paper include: Giacomo Lanzoni, Ph.D., assistant research professor, DRI; Elina Linetsky, Ph.D., DRI director of quality assurance and regulatory affairs; Diego Correa, M.D., Ph.D., assistant professor (Research) Dept. of Orthopaedics and the DRI, adjunct assistant professor of biology at Case Western Reserve University; Shari Messinger Cayetano, Ph.D., associate professor of Public Health Sciences at the Miller School; Roger A. Alvarez, D.O., M.P.H., a pulmonologist with UHealth Pulmonary and Sleep Medicine; Antonio C Marttos, M.D., a UHealth general surgeon; Ana Alvarez Gil, DRI; Raffaella Poggioli, M.D., DRI; Phillip Ruiz, M.D., Ph.D., department of Surgery at the Miller School and the UHealth Anatomic Pathology department; Khemraj Hirani, M.Pharm., Ph.D., R.Ph., CCRP, CIP, RAC, M.B.A., director of regulatory affairs and quality assurance at the DRI; Crystal A. Bell, department of medicine at the Miller School; Halina Kusack, department of Medicine, Miller School; Lisa Rafkin, research assistant professor, DRI; Rodolfo Alejandro, M.D., professor of Medicine at the Miller School, co-director of the Cell Transplant Center, and director/attending physician of the Clinical Cell Transplant Program at the DRI; David Baidal, M.D., assistant professor of Medicine in the division of Endocrinology, Diabetes & Metabolism at the Miller School and member of the DRI’s Clinical Islet Transplant Program; Andrew Pastewski, M.D., Jackson Health System; Kunal Gawri, Miller School and University of Miami Health System; Dimitrios Kouroupis, postdoctoral research fellow at the Miller School; Clarissa Leñero, DRI; Alejandro M.A. Mantero, Ph.D., lead research analyst, department of Health Sciences at the Miller School; Xiaojing Wang, DRI; Luis Roque, DRI; Burlett Masters, DRI; Norma S. Kenyon, Ph.D., deputy director and the Martin Kleiman professor of Surgery, Microbiology and Immunology and Biomedical Engineering at the DRI; Enrique Ginzburg, M.D., chief of Surgery at University of Miami Hospital and Trauma Medical Director at Jackson South Community Hospital; Xiumin Xu, DRI; Jianming Tan, M.D., Ph.D., Fuzhou General Hospital, Fujian, China; Arnold I. Caplan, Ph.D., professor of Biology at Case Western Reserve University; and Marilyn Glassberg, M.D., division chief of Pulmonary Medicine, Critical Care and Sleep Medicine at the University of Arizona College of Medicine.







Putting 2+2 together and getting 4 is easy. Too bad our governments and medical professions hasn’t that ability to figure out how to put 2+2 together as they search for answers to our medical problems! Here we are, a million people and more dead and millions more suffering as the people of the world and their nation’s economies are suffering through a coronavirus epidemic, a simple known fact has yet to be investigated thoroughly as the cure for all our problems with the coronavirus. The umbilicord of a birthing mother has all the ingredients needed to create a human being as well as having many ingredients that can be used by others that are ill to overcome their illnesses! Here we are a year into this epidemic and stem cells are yet to be used to treat people sickened by the coronavirus as people are dying every second from it! Here we are testing the thought when we should be administering the idea for the cure! What is wrong with you people? Can’t you see you have the cure in your hands as you watch your loved ones die and suffer? Can’t you just inject everyone that has coronavirus with stem cells from umbilical cord especially those that are seriously ill? Why not immediately try to do that to see what happens? Many of those that you refuse to inject with stem cells are going to die if you don’t try something so why not try stem cells immediately! Why wait for our super slow poke government of ours and our slow to get our cure medical community to get their intelligence up to par with the facts of the matter which is that umbilical stem cells cure some illnesses and just DEMAND that YOUR doctor IMMEDIATELY inject umbilical stem cells into your loved ones because YOU are the ONLY ones who are going to be able to save your loved ones from the inadequacies of our governments and our medical community to be able to do what needs to be done! I believe you can DEMAND that your doctor administer umbilical cord stem cells now without getting any special permission to do so so DEMAND that your doctor do so or get another doctor to do it if your doctor won’t! All the deaths and sufferings of all kinds need not ever happened if we just let our moms give us our cure for the coronavirus with their discarded, disposed umbilicord cord that holds our cure!!!!!!!!!!!!!!!! Wake up everybody and realize the cure is before your eyes! Why let anyone else die or suffer?
If there is enough study to see it helps out with the virus and others aswell.stop the suffering and get it done
Years ago I checked out stem cells for COPD (emphysema) and found it would cost $15,000, plus air fare to California for the procedure (no charge for the room and bed). As prices always go up, I would hazard a guess that it would be over $20,000 now. Some people can’t even afford to go to a doctor, so silently die from Covis-19. It will definitely be a cure for the rich!
cures for the rich…..sad
Please provide additional information regarding this stem cell therapy for COVID ARDS patients.
Treatment availability, cost, success rate, etc
Good Morning Ron McCure,
I am a 86 yr old man with Bronchiectasis, which I have had for several years after a
bout of Nocardia. I ended up with Bronchiectasis. I have a lot of mucus and trouble
breathing after short walks. I wake up every three to four hours a night with shortness of breath and need to use an inhaler. I am thinking of going to Panama City, Panama for stem cell treatment. They treat you with MSC stem cells. However they don’t guarantee the procedure will help with lung conditions. It is quite expensive. But I am desperate.
Your article is so true and your doing a fantastic job in relating this to the public.
So I guess this would not be legal in California? Which state are you located in.
Whenever this exists I would travel there for sure. Your article should be on every News
Station!
Best Regards,