Researchers have developed an efficient artificial photosynthetic system that mimics a natural chloroplast, converting carbon dioxide in water into methane using light. This breakthrough could contribute to carbon neutrality by creating carbon-neutral fuel, overcoming past challenges with photosensitizer stability and selectivity in water.
A joint research team from the City University of Hong Kong (CityU) and collaborators recently developed a stable artificial photocatalytic system that is more efficient than natural photosynthesis. The newly developed system, which replicates a natural chloroplast, is capable of transforming carbon dioxide in water into methane, a useful fuel, very efficiently using light. This represents a significant breakthrough with potential contributions toward achieving carbon neutrality.
For context, photosynthesis is the mechanism through which chloroplasts in plants and certain organisms utilize sunlight, water, and carbon dioxide to produce food or energy. Over the past several decades, numerous researchers have endeavored to create synthetic photosynthesis processes with the objective of converting carbon dioxide into carbon-neutral fuel.
“However, it is difficult to convert carbon dioxide in water because many photosensitizers or catalysts degrade in water,” explained Professor Ye Ruquan, Associate Professor in the Department of Chemistry at CityU, one of the leaders of the joint study. “Although artificial photocatalytic cycles have been shown to operate with higher intrinsic efficiency, the low selectivity and stability in water for carbon dioxide reduction have hampered their practical applications.”
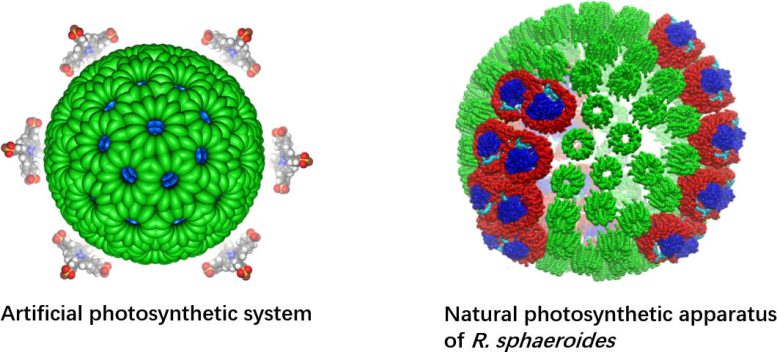
A hierarchical self-assembly photocatalytic system (left) mimics the natural photosynthesis apparatus of a purple bacteria, called Rhodobacter sphaeroides (right), achieving 15% solar-to-fuel efficiency when converting carbon dioxide into methane. Credit: (left) Professor Ye Ruquan’s research group / City University of Hong Kong and (right) Biophysical Journal, 99:67-75, 2010
In the latest study, the joint-research team from CityU, The University of Hong Kong (HKU), Jiangsu University, and the Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences overcame these difficulties by using a supramolecular assembly approach to create an artificial photosynthetic system. It mimics the structure of a purple bacteria’s light-harvesting chromatophores (i.e. cells that contain pigment), which are very efficient at transferring energy from the sun.
The core of the new artificial photosynthetic system is a highly stable artificial nanomicelle – a kind of polymer that can self-assemble in water, with both a water-loving (hydrophilic) and a water-fearing (hydrophobic) end. The nanomicelle’s hydrophilic head functions as a photosensitizer to absorb sunlight, and its hydrophobic tail acts as an inducer for self-assembly.
When it is placed in water, the nanomicelles self-assemble due to intermolecular hydrogen bonding between the water molecules and the tails. Adding a cobalt catalyst results in photocatalytic hydrogen production and carbon dioxide reduction, resulting in the production of hydrogen and methane.

Professor Ye Ruquan (front row, center), Associate Professor in the Department of Chemistry and his research team at City University of Hong Kong. Credit: City University of Hong Kong
Using advanced imaging techniques and ultrafast spectroscopy, the team unveiled the atomic features of the innovative photosensitizer. They discovered that the special structure of the nanomicelle’s hydrophilic head, along with the hydrogen bonding between water molecules and the nanomicelle’s tail, make it a stable, water-compatible artificial photosensitizer, solving the conventional instability and water-incompatibility problem of artificial photosynthesis. The electrostatic interaction between the photosensitizer and the cobalt catalyst, and the strong light-harvesting antenna effect of the nanomicelle improved the photocatalytic process.
In the experiment, the team found that the methane production rate was more than 13,000μmol h−1 g−1, with a quantum yield of 5.6% over 24 hours. It also achieved a highly efficient solar-to-fuel efficiency rate of 15%, surpassing natural photosynthesis.
Most importantly, the new artificial photocatalytic system is economically viable and sustainable, as it doesn’t rely on expensive precious metals. “The hierarchical self-assembly of the system offers a promising bottom-up strategy to create a precisely controlled, high-performance artificial photocatalytic system based on cheap, Earth-abundant elements, like zinc and cobalt porphyrin complexes,” said Professor Ye.
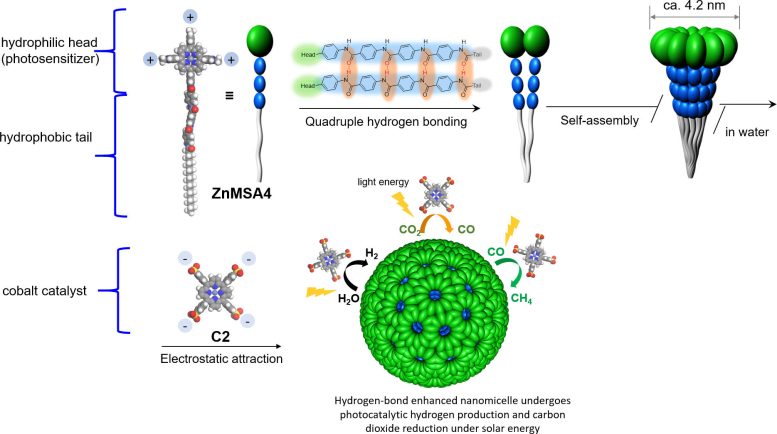
Formation of a hydrogen-bond enhanced nanomicelle and its hydrogen production and carbon dioxide reduction under solar energy. Credit: Professor Ye Ruquan’s research group / City University of Hong Kong
Professor Ye said he believes the latest discovery will benefit and inspire the rational design of future photocatalytic systems for carbon dioxide conversion and reduction using solar energy, contributing to the goal of carbon neutrality.
Reference: “Artificial spherical chromatophore nanomicelles for selective CO2 reduction in water” by Junlai Yu, Libei Huang, Qingxuan Tang, Shang-Bo Yu, Qiao-Yan Qi, Jiangshan Zhang, Danying Ma, Yifei Lei, Jianjun Su, Yun Song, Jean-Charles Eloi, Robert L. Harniman, Ufuk Borucu, Long Zhang, Minghui Zhu, Feng Tian, Lili Du, David Lee Phillips, Ian Manners, Ruquan Ye and Jia Tian, 18 May 2023, Nature Catalysis.
DOI: 10.1038/s41929-023-00962-z
The first authors are Dr. Yu Junlai, from the Shanghai Institute of Organic Chemistry, and Dr. Huang Libei, CityU Ph.D. The corresponding authors are Professor Ye, Professor David Lee Philips, from HKU, Professor Du Lili, from Jiangsu University, and Professor Tian Jia, from the Shanghai Institute of Organic Chemistry.
The study was supported by various funding sources, including the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Fund, the Shenzhen Science and Technology Program, and the Hong Kong Research Grant Council.








Great. A new way to transform a greenhouse gas into a much more effective greenhouse gas.
Kind of a little upside down and backwards. How about “Don’t burn it in the first place?”. Then you don’t have to worry about cleaning it up.
One should look at the entire system when calculating efficiency. The artificial chloroplasts have to be created with energy, probably put into special greenhouses that require energy to be manufactured, and periodically maintained/repaired.
Plants, on the other hand, will naturally reproduce, repair minor damage, and use free air and sunlight to produce cellulose and sugars.
I think that a better approach might be to do gene modification to produce plants that are more efficient at doing what they do naturally.
If this can be scaled up to draw massive amounts of CO2 from the oceans, and if the resulting methane can then be processed into synthetic fuel, it would be a good way to reach carbon neutrality. I have my doubts, however, that such a large scale endeavor will ever find enough political will to get any traction.
As it is, it will require energy to circulate air past the synthetic chloroplasts, which isn’t free. Extracting CO2 from water will probably require even more energy. One can heat water to drive off CO2, but calcium carbonate is less soluble in warm water, causing it to precipitate out. One then has the problem of what to do with precipitated carbonate. Trucking it off and burying it requires energy. Meanwhile, the original water will be warmer than it was and have a higher pH. If it is dumped back in the ocean what will happen to the ecosystem it came from?
It seems the people most accepting of the paradigm of anthropogenic-caused global warming, and supportive of carbon neutrality, are the least knowledgeable of Earth science.
The CO2 goes in, and CH4 goes out. Water takes up CO2 easily, while CH4 (methane) is barely soluble. This needs no energy, but maybe for bubbling CO2 (or air) through. There is no need to convert CH4 to synthetic fuel, because this the same stuff as “natural gas”. Plenty of it are used for domestic heating, and by the industry. When combusted, it ends up as CO2 again. No additional CO2 produced. A part of the energy from the sun can be needed for the synthesis of the catalysts.