The drug protects mice from sudden death that was caused by a rupture of a major blood vessel.
The drug could pave the way for treatments for those who are at risk of sudden rupture due to abdominal aortic aneurysms.
A study conducted by scientists at Washington University School of Medicine in St. Louis shows that an experimental drug therapy protects mice against sudden death brought on by the rupture of a major blood vessel in the abdomen.
The research, which was published in the journal Biomaterials Advances, could lead to a new approach to treating abdominal aortic aneurysms, a condition in which the wall of the abdominal aorta, a major blood vessel that carries blood from the heart to the rest of the body, weakens and bulges outward. Without notice, the weak spot can begin to leak blood or possibly burst, causing a serious emergency that, if not treated quickly, almost always ends in death. The larger the aneurysm, the more probable it may burst unexpectedly.
“When people are identified with a medium or small aneurysm, we monitor them,” said senior author Christine T. N. Pham, MD, the Guy and Ella Mae Magness Professor of Medicine and director of the Division of Rheumatology. “Large aneurysms can be repaired surgically, but for smaller aneurysms, there’s no treatment other than waiting for them to get to a size that can be repaired surgically. Our findings in mice illustrate a potentially relevant therapy that could prevent rupture of the aneurysm.”
Pham treats patients at Barnes-Jewish Hospital and the Veterans Affairs Medical Center in St. Louis.
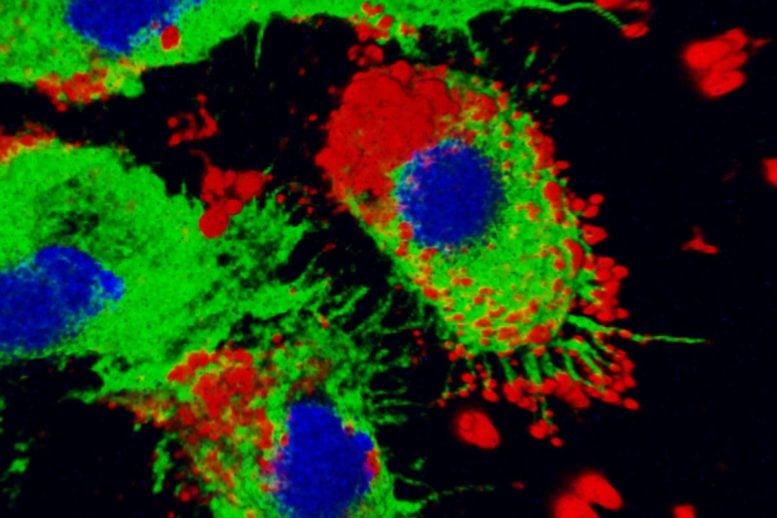
Nanoparticles (red) are taken up by immune cells (green with blue nuclei). Researchers at Washington University School of Medicine in St. Louis have shown that an experimental nanoparticle-based drug therapy protects mice from sudden death due to the rupture of a major blood vessel in the abdomen, pointing the way toward a new strategy for treating deadly abdominal aortic aneurysms. Credit: Huimin Yan/Washington University
Every year, around 200,000 individuals in the United States are diagnosed with abdominal aortic aneurysm, also known as triple A, the majority of whom are older males who smoke. Such aneurysms often don’t show any symptoms until they abruptly and catastrophically rupture, killing 15,000 people annually in the United States alone. All men aged 65 to 75 who have ever smoked should get ultrasound scans to test for triple A, according to the U.S. Preventive Services Task Force, a group of independent experts in disease prevention and evidence-based medicine supported by the U.S. Department of Health and Human Services.
For decades, scientists have known that inflammation in blood vessels causes the progression of triple A, but efforts to treat the disease with immunosuppressive therapies have failed. The immune system is a critical component of the body’s infection defenses. It is difficult to strike a careful balance between reducing inflammation in the aorta sufficiently to prevent aneurysms from worsening without suppressing the immune system in the rest of the body to the point where a person becomes susceptible to severe infections.
In this study, the researchers used nanoparticles to deliver anti-inflammatory payloads directly to inflamed blood vessels. The nanoparticle is based on a fragment of a protein called melittin and optimized to carry the payload: small bits of RNA. The modified protein fragment forms a complex with RNA that, when given to the mice, accumulates primarily in inflamed tissues. There, the protein fragment unloads the bits of RNA and assists their entry into the cells’ main compartment, where the RNA suppresses inflammation by interfering with the expression of an important inflammatory protein, NF-kappaB.
Co-author Samuel A. Wickline, MD, formerly of Washington University School of Medicine and now a professor at the University of South Florida and the chief scientific officer at the biotechnology company Altamira Therapeutics, created the basic version of the nanoparticle while at Washington University. This study involves an optimized version of the nanoparticle that was created by Wickline, Pham, and their Washington University co-authors Hua Pan, Ph.D., an associate professor of medicine, and first author Huimin Yan, MD, Ph.D., a staff scientist.
The researchers used the nanoparticles to carry so-called small interfering RNAs (siRNAs) targeting two subunits of NF-kappaB: p50 and p65. The researchers studied male mice that developed a triple A-like condition that ruptures about half the time. They treated the mice with nanoparticles containing p50 siRNA, p65 siRNA, or an irrelevant siRNA for comparison. Suppressing p50 did not halt the progression of the aneurysms, but significantly increased the mice’s chances of survival, from 53% to 85%. Treatment also delayed the onset of rupture, from day seven to day twelve. In contrast, suppressing p65 did not have a significant effect.
“Optimization of the nanoparticle allowed us to use a fraction of the previously established dose of siRNA, which means we can achieve a therapeutic effect at a level that is less likely to cause adverse effects,” Pan said. “By targeting p50 and p65 separately, we pieced out the individual contributions of the different subunits and found the one (p50) that we think will be more protective with less potential adverse effects. Altogether, these results are very encouraging. They suggest that it may be possible to develop a therapy to reduce the risk of rupture and death from triple A without unacceptable adverse effects.”
Wickline is the principal investigator and Pham the Washington University site lead on a Small Business Technology Transfer grant from the National Institutes of Health (NIH) involving the original nanoparticle technology created by Wickline and his team at Washington University. The grant supports a project to develop and commercialize the technology as a treatment for inflammatory disease in collaboration with Altamira Therapeutics.
“For that grant, we are looking at rheumatoid arthritis, not triple A,” Pham said. “But once you have the technology approved for one disease, it is a lot easier to apply it to other diseases. I’m hopeful that one day, in the not-too-distant future, we’ll have a treatment to offer people to stabilize the aneurysm, reducing the risk of rupture and sudden death. The technology is still being tested, but there’s more hope now.”
Reference: “Peptide-siRNA nanoparticles targeting NF-κB p50 mitigate experimental abdominal aortic aneurysm progression and rupture” by Huimin Yan, Ying Hua, Antonina Akk, Samuel A. Wickline, Hua Pan and Christine T.N. Pham, 6 July 2022, Biomaterials Advances.
DOI: 10.1016/j.bioadv.2022.213009
The study was funded by the National Institutes of Health (NIH) and the Department of Veterans’ Affairs.







Be the first to comment on "New Experimental Drug Protects Against Sudden Death"