
University of Illinois Chicago researchers have identified a mechanism where heart cell enzymes can prevent damage from chemotherapy drugs. This discovery holds potential for personalized medicine approaches to chemotherapy, potentially enhancing heart cell protection and paving the way for future research into heart disease and other conditions.
Researchers from the University of Illinois Chicago have discovered a new process by which enzymes can help reduce heart damage in chemotherapy patients.
These enzymes, typically located in a cell’s mitochondria—the energy-producing powerhouses—are observed to migrate to the cell’s nucleus when the heart cells encounter stress from specific chemotherapy drugs. The relocation of these enzymes appears to aid in the survival of these cells. The paper was published on July 19 in the journal Nature Communications.
Rise of Cardio-Oncology and Its Challenges
“As chemotherapy has become more and more effective, we have more and more cancer survivors. But the tragic part is that a lot of these survivors now have problems with heart failure,” explained co-senior author Sang Ging Ong, assistant professor of pharmacology and medicine.
This unfortunate trend has resulted in the emergence of a new field, cardio-oncology, which primarily investigates the mechanisms by which chemotherapy drugs harm heart cells’ mitochondria. The research team sought to explore an alternative perspective: Why are some patients’ hearts immune to damage? Could there be unique aspects of their cells that provide this protection?
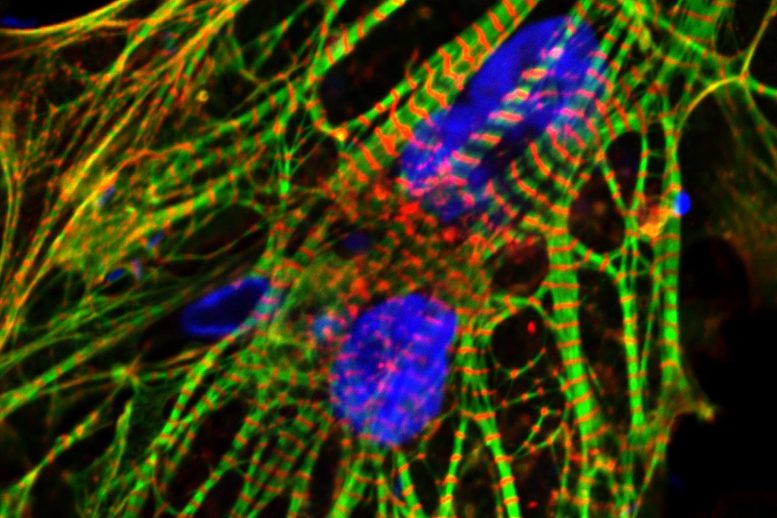
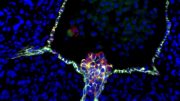
Human induced pluripotent stem cell-derived heart cells (cardiomyocytes) show the cardiac proteins actinin (red) and troponin T (green) as well as the nucleus (blue). Credit: Image adapted from the research paper
Unraveling the Mechanisms of Heart Cell Protection
First, the team discovered that when the heart cells were stressed by chemotherapy, the mitochondrial enzymes moved into the cell’s nucleus — an unusual phenomenon. However, the researchers were unsure whether this enzyme migration was responsible for the cell’s damage or its protection, explained Dr. Jalees Rehman, co-senior author and head of the UIC Department of Biochemistry and Molecular Genetics.
“We really didn’t know which way it would go,” he said.
In order to clarify this ambiguity, the team created versions of the enzymes that specifically targeted the nucleus, bypassing the mitochondria. They found that this intentional enzyme relocation fortified the cells, effectively enhancing their survival. This protective mechanism was observed in both heart cells derived from human stem cells and in mice subjected to chemotherapy.
“This seems to be a new mechanism by which heart cells can defend themselves against chemotherapy damage,” said Rehman, who is also a member of the University of Illinois Cancer Center.
New Clinical Possibilities and Future Research
This finding implies new clinical potential. Physicians could analyze individual patients to determine if their heart cells, created from personalized stem cells, could protect themselves from chemotherapy by moving their enzymes from their mitochondria into the cell’s nucleus. This process would involve drawing blood from the patient, creating stem cells from the blood cells, and then using these personalized stem cells to generate heart cells genetically identical to the patient’s own heart cells.
“Assessing the injury caused by chemotherapy and the enzyme movement from the mitochondria into the nucleus of those heart cells in a lab would help determine what the patient’s likely response would be to chemotherapy,” Rehman said.
For patients with inadequate protection, it may be possible to enhance this protection by increasing the enzyme movement and fortifying the heart cells.
The researchers are excited to conduct further studies to determine if this approach could help prevent heart damage from other conditions, such as high blood pressure and heart attacks, and whether it could be applied to other cells, like those in blood vessels.
Reference: “Nuclear translocation of mitochondrial dehydrogenases as an adaptive cardioprotective mechanism” by Shubhi Srivastava, Priyanka Gajwani, Jordan Jousma, Hiroe Miyamoto, Youjeong Kwon, Arundhati Jana, Peter T. Toth, Gege Yan, Sang-Ging Ong and Jalees Rehman, 19 July 2023, Nature Communications.
DOI: 10.1038/s41467-023-40084-5
The other authors on the paper are Shubhi Srivastava, Priyanka Gajwani, Jordan Jousma, Hiroe Miyamoto, Youjeong Kwon, Arundhati Jana, Peter Toth and Gege Yan, all at UIC’s College of Medicine. The research was funded by grants from the National Institutes of Health and the American Heart Association.




Be the first to comment on "New Frontier in Cardio-Oncology: Powerhouse Proteins Protect Heart Cells From Chemotherapy Damage"