The findings could lead to better, more personalized immunotherapies.
According to a recent study, immune responses could potentially be enhanced even in patients who do not exhibit any visible clinical response.
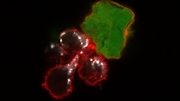
A research team has, for the first time, identified and analyzed the process by which immune cells “see” and react to cancer cells. This provides valuable insights into why certain treatments may work for some patients but not for others.
The scientists leading the research at the UCLA Jonsson Comprehensive Cancer Center anticipate that their discoveries will result in improved and more customized immunotherapies, even for patients who seem unresponsive to treatment.
“This is an important step forward in our understanding of what the T-cell responses see in the tumor and how they change over time while they are in the tumor and in circulation in the blood, searching for new tumor cells to attack,” said Cristina Puig-Saus, Ph.D., a UCLA Jonsson Comprehensive Cancer Center researcher, adjunct assistant professor of medicine at UCLA, and the first author of a study in Nature.
“The deeper understanding of how the T-cell responses clear metastatic tumor masses will help us design better treatments and engineer T cells in multiple ways to mimic them,” she said.
The researchers adapted advanced gene-editing technology to make unprecedented observations about immune responses in patients with metastatic melanoma receiving anti-PD-1 “checkpoint inhibitor” immunotherapy. Although immune cells called T cells have the ability to detect mutations in cancer cells and eliminate them, leaving normal cells unharmed, cancer cells often evade the immune system. Checkpoint inhibitors are designed to improve the T cells’ ability to recognize and attack cancer cells.
“With this work, we can know exactly what the immune system of a particular patient recognized in their cancer to differentiate it from normal cells and attack it,” said Antoni Ribas, M.D., Ph.D., a UCLA Jonsson Comprehensive Cancer Center researcher, professor of medicine at UCLA, a co-senior author of the study.
The investigators showed that when the immunotherapy is effective, it directs a diverse repertoire of T cells against a small group of selected mutations in a tumor. These T-cell responses expand and evolve during the course of treatment, both within the tumor and in the bloodstream. Patients for whom the therapy fails also present a T-cell response against a similarly reduced number of mutations in the tumor, but those immune responses are less focused, and they do not expand during treatment.
“This study demonstrates that patients without response to therapy still induce a tumor-reactive T-cell response,” Puig-Saus said. “These T cells could potentially be isolated and their immune receptors used to genetically modify a larger number of T cells to redirect them against the patient’s tumor. These T cells could be expanded in culture and reinfused into the patients to treat their tumors.”
In the 11 patients studied, seven had a response to PD-1 blockade; four did not. The number of mutations in the tumors ranged between 3,507 and 31. Despite this wide range, the number of mutations seen by tumor-reactive T cells ranged between 13 and one. In patients with clinical benefit from the therapy, the responses were diverse, with a range between 61 and seven different mutation-specific T cells isolated in the blood and the tumor. In contrast, in the patients lacking a response to therapy, the researchers only identified between 14 and two different T cells.
Also, in patients responding to treatment, the researchers were able to isolate tumor-reactive T cells in blood and tumors throughout treatment, but in patients without a response, the T cells were not recurrently detected. Still, the study showed that immune receptors from the T cells isolated from all patients – regardless of response or not – redirected the specificity of immune cells against the tumor, producing antitumor activity.
The work to characterize T-cell activity in patients with and without a clinical response was made possible through the creation of a new technique using sophisticated technology to isolate mutation-reactive T cells from blood and tumor samples. It builds on technology developed through a collaboration with Ribas, James Heath, Ph.D., president of the Institute for Systems Biology in Seattle, and David Baltimore, Ph.D., Nobel laureate, emeritus professor at Caltech, and a member of the UCLA Jonsson Comprehensive Cancer Center.
As previously published in Nature and presented at the Society for Immunotherapy of Cancer (SITC) 2022 last November, the technology was further developed by PACT Pharma, using CRISPR gene editing to insert genes into immune cells to efficiently redirect them to recognize mutations in a patient’s own cancer cells.
“With this technique, we generated large numbers of T cells expressing the immune receptors from the mutation-reactive T cells isolated from each patient. We used these cells to characterize the reactivity of the immune receptors against the patient’s own cancer cells,” Ribas said. “The new technologies allow us to study these rare immune cells that are the mediators of immune responses to cancer.”
Reference: “Neoantigen-targeted CD8+ T cell responses with PD-1 blockade therapy” by Cristina Puig-Saus, Barbara Sennino, Songming Peng, Clifford L. Wang, Zheng Pan, Benjamin Yuen, Bhamini Purandare, Duo An, Boi B. Quach, Diana Nguyen, Huiming Xia, Sameeha Jilani, Kevin Shao, Claire McHugh, John Greer, Phillip Peabody, Saparya Nayak, Jonathan Hoover, Sara Said, Kyle Jacoby, Olivier Dalmas, Susan P. Foy, Andrew Conroy, Michael C. Yi, Christine Shieh, William Lu, Katharine Heeringa, Yan Ma, Shahab Chizari, Melissa J. Pilling, Marc Ting, Ramya Tunuguntla, Salemiz Sandoval, Robert Moot, Theresa Hunter, Sidi Zhao, Justin D. Saco, Ivan Perez-Garcilazo, Egmidio Medina, Agustin Vega-Crespo, Ignacio Baselga-Carretero, Gabriel Abril-Rodriguez, Grace Cherry, Deborah J. Wong, Jasreet Hundal, Bartosz Chmielowski, Daniel E. Speiser, Michael T. Bethune, Xiaoyan R. Bao, Alena Gros, Obi L. Griffith, Malachi Griffith, James R. Heath, Alex Franzusoff, Stefanie J. Mandl and Antoni Ribas, 8 March 2023, Nature.
DOI: 10.1038/s41586-023-05787-1
The study was funded by the National Institutes of Health and PACT Pharma.



Be the first to comment on "New Potential for Immunotherapy: Scientists Shed Light on How Immune Cells Respond to Cancer Cells"