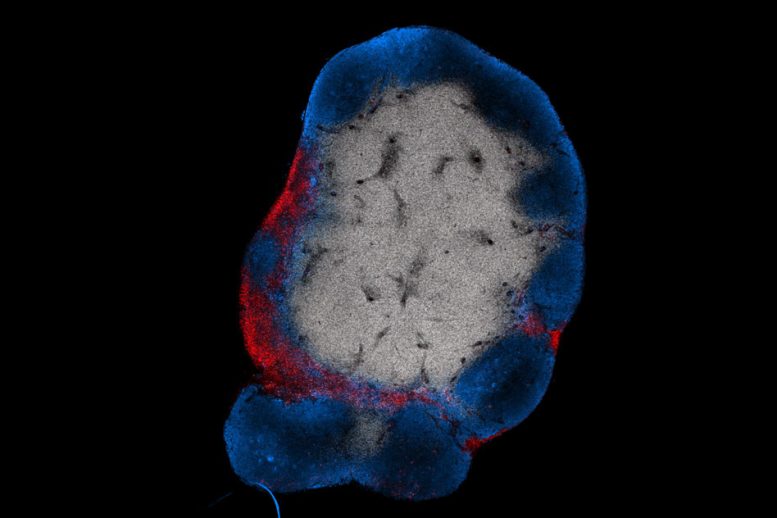
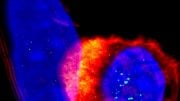
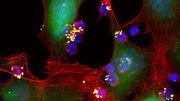
MIT engineers have devised a way to stimulate T cells (shown in red) to attack tumors by activating them with a vaccine that accumulates in the lymph nodes. B cells in the lymph nodes are labeled in blue. Image: Leyuan Ma and Jason Chang
A promising new way to treat some types of cancer is to program the patient’s own T cells to destroy the cancerous cells. This approach, termed CAR-T cell therapy, is now used to combat some types of leukemia, but so far it has not worked well against solid tumors such as lung or breast tumors.
MIT researchers have now devised a way to super-charge this therapy so that it could be used as a weapon against nearly any type of cancer. The research team developed a vaccine that dramatically boosts the antitumor T cell population and allows the cells to vigorously invade solid tumors.
In a study of mice, the researchers found that they could completely eliminate solid tumors in 60 percent of the animals that were given T-cell therapy along with the booster vaccination. Engineered T cells on their own had almost no effect.
“By adding the vaccine, a CAR-T cell treatment which had no impact on survival can be amplified to give a complete response in more than half of the animals,” says Darrell Irvine, who is the Underwood-Prescott Professor with appointments in Biological Engineering and Materials Science and Engineering, an associate director of MIT’s Koch Institute for Integrative Cancer Research, a member of the Ragon Institute of MGH, MIT, and Harvard, and the senior author of the study.
Leyuan Ma, an MIT postdoc, is the lead author of the study, which appears in the July 11 online edition of Science.
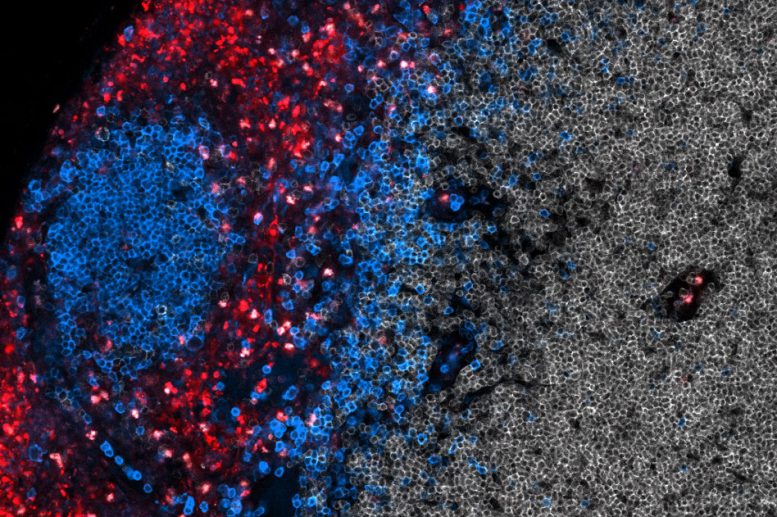
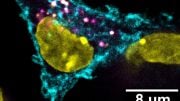
A zoomed in image of T cells (shown in red) being activated with a vaccine that accumulates in the lymph nodes. B cells in the lymph nodes are labeled in blue. Image: Leyuan Ma and Jason Chang
Targeting tumors
So far, the FDA has approved two types of CAR-T cell therapy, both used to treat leukemia. In those cases, T cells removed from the patient’s blood are programmed to target a protein, or antigen, found on the surface of B cells. (The “CAR” in CAR-T cell therapy is for “chimeric antigen receptor.”)
Scientists believe one reason this approach hasn’t worked well for solid tumors is that tumors usually generate an immunosuppressive environment that disarms the T cells before they can reach their target. The MIT team decided to try to overcome this by giving a vaccine that would go to the lymph nodes, which host huge populations of immune cells and stimulate the CAR-T cells there.
“Our hypothesis was that if you boosted those T cells through their CAR receptor in the lymph node, they would receive the right set of priming cues to make them more functional so they’d be resistant to shutdown and would still function when they got into the tumor,” Irvine says.
To create such a vaccine, the MIT team used a trick they had discovered several years ago. They found that they could deliver vaccines more effectively to the lymph nodes by linking them to a fatty molecule called a lipid tail. This lipid tail binds to albumin, a protein found in the bloodstream, allowing the vaccine to hitch a ride directly to the lymph nodes.
In addition to the lipid tail, the vaccine contains an antigen that stimulates the CAR-T cells once they reach the lymph nodes. This antigen could be either the same tumor antigen targeted by the T cells or an arbitrary molecule chosen by the researchers. For the latter case, the CAR-T cells have to be re-engineered so that they can be activated by both the tumor antigen and the arbitrary antigen.
In tests in mice, the researchers showed that either of these vaccines dramatically enhanced the T-cell response. When mice were given about 50,000 CAR-T cells but no vaccine, the CAR-T cells were nearly undetectable in the animals’ bloodstream. In contrast, when the booster vaccine was given the day after the T-cell infusion, and again a week later, CAR-T cells expanded until they made up 65 percent of the animals’ total T-cell population, two weeks after treatment.
This huge boost in the CAR-T cell population translated to the complete elimination of glioblastoma, breast, and melanoma tumors in many of the mice. CAR-T cells given without the vaccine had no effect on tumors, while CAR-T cells given with the vaccine eliminated tumors in 60 percent of the mice.
Long-term memory
This technique also holds promise for preventing tumor recurrence, Irvine says. About 75 days after the initial treatment, the researchers injected tumor cells identical to those that formed the original tumor, and these cells were cleared by the immune system. About 50 days after that, the researchers injected slightly different tumor cells, which did not express the antigen that the original CAR-T cells targeted; the mice could also eliminate those tumor cells.
This suggests that once the CAR-T cells begin destroying tumors, the immune system is able to detect additional tumor antigens and generate populations of “memory” T cells that also target those proteins.
“If we take the animals that appear to be cured and we rechallenge them with tumor cells, they will reject all of them,” Irvine says. “That is another exciting aspect of this strategy. You need to have T cells attacking many different antigens to succeed, because if you have a CAR-T cell that sees only one antigen, then the tumor only has to mutate that one antigen to escape immune attack. If the therapy induces new T-cell priming, this kind of escape mechanism becomes much more difficult.”
While most of the study was done in mice, the researchers showed that human cells coated with CAR antigens also stimulated human CAR-T cells, suggesting that the same approach could work in human patients. The technology has been licensed to a company called Elicio Therapeutics, which is seeking to test it with CAR-T cell therapies that are already in development.
“There’s really no barrier to doing this in patients pretty soon, because if we take a CAR-T cell and make an arbitrary peptide ligand for it, then we don’t have to change the CAR-T cells,” Irvine says. “I’m hopeful that one way or another this can get tested in patients in the next one to two years.”
The research was funded by the National Institutes of Health, the Marble Center for Cancer Nanomedicine, Johnson and Johnson, and the National Institute of General Medical Sciences.
Reference: “Enhanced CAR–T cell activity against solid tumors by vaccine boosting through the chimeric receptor” by Leyuan Ma, Tanmay Dichwalkar, Jason Y. H. Chang, Benjamin Cossette, Daniel Garafola, Angela Q. Zhang, Michael Fichter, Chensu Wang, Simon Liang, Murillo Silva, Sudha Kumari, Naveen K. Mehta, Wuhbet Abraham, Nikki Thai, Na Li, K. Dane Wittrup and Darrell J. Irvine, 12 July 2019, Science.
DOI: 10.1126/science.aav8692


Be the first to comment on "New Super-Charged Treatment Boosts T-Cell Therapy"